- Research article
- Open access
- Published:
Heterologous versus homologous COVID-19 booster vaccinations for adults: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials
BMC Medicine volume 22, Article number: 263 (2024)
Abstract
Background
To combat coronavirus disease 2019 (COVID-19), booster vaccination strategies are important. However, the optimal administration of booster vaccine platforms remains unclear. Herein, we aimed to assess the benefits and harms of three or four heterologous versus homologous booster regimens.
Methods
From November 3 2022 to December 21, 2023, we searched five databases for randomised clinical trials (RCT). Reviewers screened, extracted data, and assessed bias risks independently with the Cochrane risk-of-bias 2 tool. We conducted meta-analyses and trial sequential analyses (TSA) on our primary (all-cause mortality; laboratory confirmed symptomatic and severe COVID-19; serious adverse events [SAE]) and secondary outcomes (quality of life [QoL]; adverse events [AE] considered non-serious). We assessed the evidence with the GRADE approach. Subgroup analyses were stratified for trials before and after 2023, three or four boosters, immunocompromised status, follow-up, risk of bias, heterologous booster vaccine platforms, and valency of booster.
Results
We included 29 RCTs with 43 comparisons (12,538 participants). Heterologous booster regimens may not reduce the relative risk (RR) of all-cause mortality (11 trials; RR 0.86; 95% CI 0.33 to 2.26; I2 0%; very low certainty evidence); laboratory-confirmed symptomatic COVID-19 (14 trials; RR 0.95; 95% CI 0.72 to 1.25; I2 0%; very low certainty); or severe COVID-19 (10 trials; RR 0.51; 95% CI 0.20 to 1.33; I2 0%; very low certainty). For safety outcomes, heterologous booster regimens may have no effect on SAE (27 trials; RR 1.15; 95% CI 0.68 to 1.95; I2 0%; very low certainty) but may raise AE considered non-serious (20 trials; RR 1.19; 95% CI 1.08 to 1.32; I2 64.4%; very low certainty). No data on QoL was available. Our TSAs showed that the cumulative Z curves did not reach futility for any outcome.
Conclusions
With our current sample sizes, we were not able to infer differences of effects for any outcomes, but heterologous booster regimens seem to cause more non-serious AE. Furthermore, more robust data are instrumental to update this review.
Background
Severe respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen that causes coronavirus disease (COVID-19). Despite the official end of the public health emergency declaration on 5 May 2023, SARS-CoV-2 continues to infect people across the world, with vaccination remaining one of the most important protective measures against COVID-19 [1, 2].
Between 31 July and 27 August 2023, more than 1.4 million new COVID-19 patients and over 1800 deaths were reported globally underscoring the need for ongoing close monitoring of circulating SARS-CoV-2 variants closely [1]. Presently, a number of variants are tracked by WHO, including two variants of interest (VOIs) (XBB.1.5 and XBB.1.16) and a number of variants under monitoring (VUMs) [1]. Significant progress in the handling of the COVID-19 epidemic has already been made as nearly every country has implemented vaccination policies, which has resulted in major reductions in the occurrence of severe disease, hospitalisations, and mortality [2].
Despite fewer severely diseased and fewer deaths worldwide today, there are concerns about reduced protection because of waning immunity and the appearance of newly emerging variants [3]. Currently, the Strategic Advisory Group of Experts on Immunisation recommends healthy adults over the age of 18 years are to receive one booster dose after primary vaccine series, whilst individuals with the greater risk of severe disease and death (older adults, pregnant persons, and people with immunocompromised conditions) are recommended an additional booster dose [4].
Using heterologous vaccine platforms can be an alternative strategy to homologous vaccine platforms to maximise booster vaccine impact in the event of limited supplies. It is unclear whether a heterologous boosting regimen may provide higher vaccine effectiveness than homologous booster vaccines. Two meta-analyses including randomised clinical trials and observational studies suggest that heterologous booster doses have a higher protection against symptomatic COVID-19 and severe COVID-19 compared with or to homologous booster doses [5, 6] whilst a ‘living meta-analysis’ also including randomised clinical trials and observational studies does not [7].
The objective of this systematic review is to compare the vaccine benefits and harms between three or four dose heterologous boosters using different vaccine platforms or intra-platform variations versus homologous booster regimens in randomised trials only to help inform public health policies.
Methods
Recognising the needs of COVID-19 vaccine research and the identification of trials on heterologous versus homologous booster regimens as an area of public health interest necessitating evidence synthesis, we performed this specific review of pairwise comparison of heterologous versus homologous boosters in randomised clinical trials. This was performed within the framework of our living systematic review, the methodology of which is thoroughly discussed elsewhere [8], and the protocol registered in PROSPERO (CRD42020178787). This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis [9] (Additional file: PRISMA checklist) and the implementation of this review followed the recommended procedures as specified in the Cochrane Handbook of Systematic Reviews of Interventions [10].
Search strategy and trial inclusion criteria
This updated review follows a two-step approach. As for the first living systematic review, the literature searches were conducted on a biweekly basis, from 3 November 2022 to 21 December 2023 using Medline, Cochrane Central Register of Controlled Trials, Embase, Latin American and Caribbean Health Sciences Literature, and Science Citation Index Expanded to identify newly published trials following the initial search strategy and eligibility criteria (for more ample information on the search strategy and study inclusion, please refer to the protocol (Additional file: Additional search Strategy]). After identifying eligible randomised clinical trials for our original research on the efficacy of all COVID-19 vaccines in relation to all-cause mortality, safety, and vaccine efficacy, we employed a specific search strategy tailored to our present research question (Additional file: Additional search strategy). As a quality control measure, we also conducted a snowball search to identify any potential missed trials [11]. All randomised clinical trials reporting on a third or fourth heterologous booster vaccine versus either a third or fourth homologous booster vaccine were included. In instances where it was not possible to determine whether the intervention arm used a heterologous or homologous booster vaccine, and no clarification was provided by the authors, the trial was excluded. Also, only full booster doses between both arms were compared, in instances when boosters between both arms only compared half doses to full doses, the trial was excluded. Trials with mixed primary series in the heterologous arm were excluded. Furthermore, trials reporting exclusively on immunogenicity, along with trials comparing different types of heterologous booster vaccines or heterologous third booster to a placebo were also excluded. Trials that included open-label cohorts with no randomisation of the participants were excluded.
Data analysis
Outcomes
The vaccine efficacy outcomes included the primary outcomes, all-cause mortality, prevention of laboratory-confirmed symptomatic COVID-19, severe symptoms associated with COVID-19, and serious adverse events (SAE) [8]. Whenever participants were noted to have (laboratory-confirmed) COVID-19 symptoms, we classified it as symptomatic COVID-19. Conversely, if participants were hospitalised due to severe COVID-19 symptoms, we defined it as severe COVID-19. Secondary outcomes were health-related quality of life and adverse events (AE) considered not serious [8]. We used the trial results reported at maximum follow-up for each specific abovementioned outcome and used intention-to-treat data if provided by the trialist.
Data extraction and risk of bias assessment
Two independent authors conducted the screening, data extraction, quality assessment, and GRADE assessment for each eligible trial following the Cochrane risk of bias tool—version 2 and the procedure described in our protocol. If three domains were assigned a ‘some concern’ assessment, then the trial was graded at ‘high risk of bias’. Any discrepancies were resolved by consensus and authors were contacted to clarify uncertainties and provide additional context, including available data stratified by older adults.
Statistical synthesis
We performed meta-analysis using STATA 17 for Windows (StataCorp, College Station, TX, USA, 2021) and analysed data with the meta command for meta-analysis. For the trial sequential analysis (TSA), we used version 0.9.5.10 beta (TSA 2017) [12]. To quantify the strength of associations between booster vaccines and vaccine efficacy and safety outcomes, we employed relative risk (RR). The risk ratio was computed by dividing the risk observed in the heterologous vaccine regimen group by the risk in the homologous vaccine regimen group, and the 95% confidence intervals (CI) for the risk ratio was used to determine the precision of the estimated associations. With a view to avoiding attributing excessive weight to the control groups in the meta-analysis, we divided both the numerator and the denominator of the control group by the number of intervention groups whenever the same control group was used in a trial to compare different intervention groups. To account for potential heterogeneity amongst the trials, random-effects DerSimonian and Laird models were applied [13, 14]. In addition, the fixed-effect meta-analysis (Mantel–Haenszel method) was assessed separately and the most conservative point estimate of the two reported [15, 16]. We also post hoc applied Peto’s odds ratio (OR) due to very few outcomes in some comparisons.
Assessment of heterogeneity within and between study groups was conducted using the Cochrane Q test, with a significance level of p < 0.1 indicating the presence of heterogeneity [10]. The I2 statistic, as described by Higgins and Thompson was employed to estimate the percentage of observed between-study variability due to heterogeneity, as opposed to chance [17]. This statistic ranges from 0 to 100%, with values of 0 to 40% representing moderate heterogeneity, 30 to 60% moderate heterogeneity, 50 to 90% substantial heterogeneity, and 75 to 100% considerable heterogeneity [10].
Furthermore, we performed a subgroup analysis based on the risk of biases to examine the effect of potential biases on the risk ratio. The variable was categorised as low risk of bias compared to some concerns/high risk of bias, allowing us to discern any differential effects on the overall results. Moreover, we conducted subgroup analyses based on the follow-up time: studies with follow-up periods of 3 months and under were compared to those with follow-up periods of above 3 months. Additionally, we compared vaccine regimens with three doses against those with four doses to explore differences in their risk ratios. As different vaccine booster platforms use distinct mechanisms to elicit immune responses [18], which may lead to varying efficacy and safety profiles [19], we also conducted a subgroup analysis to compare differences in risk ratios between boosters with different vaccine platforms, including inactivated, protein-based, viral vectored, and mRNA-based boosters. Furthermore, we investigated the variation in risk ratios for vaccine efficacy outcomes between trials from 2023 and those from 2022, thereby allowing us to consider the potential influence of the predominance of XBB subvariants towards the end of 2022 and 2023. Also, we conducted a subgroup analysis by immunocompromised status as immunocompromised individuals may not have a robust immune response to COVID-19 vaccines compared to those without an immunocompromised condition [20]. Initially, our plan was to conduct a subgroup analysis by categorising adults into younger and older age groups; however, we were constrained by the absence of disaggregated data. Additionally, as an increase in inoculation interval times may impact vaccine efficacy and possibly safety outcomes [21], we aimed to investigate the impact of different inoculation interval times on vaccine efficacy and safety outcomes using a 12-week cutoff [22]. Nevertheless, inconsistent reporting and a lack of interpretable data due to large ranges of inoculation intervals prevented us from conducting these planned subgroup analyses. To capture more recent trials comparing vaccine valency, monovalent vaccine boosters to multivalent vaccine boosters (bivalent and tetravalent vaccine boosters) using heterologous and homologous vaccine boosters, we have also conducted a subgroup analysis. By conducting these subgroup analyses, we aimed to assess the differential effect on risk ratios and their associated heterogeneity.
We conducted the TSAs to control risks of type I and type II errors [23,24,25]. To assess publication bias, a visual inspection of the funnel plots was conducted and the Egger statistical test performed when an outcome had at least 10 trials [10].
Summary of findings and assessment of certainty
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) profiler Guideline Development Tool to create the summary of findings tables (GRADEpro GDT https://www.gradepro.org/). We created a summary of findings tables including each of the prespecified outcomes (all-cause mortality, vaccine efficacy, serious adverse events, health-related quality of life, and non-serious adverse events) (Table 1: GRADE assessment). We used the five GRADE considerations (bias risk of the trials, consistency of effect, imprecision, indirectness, and publication bias). We assessed imprecision using trial sequential analysis [8, 26, 27].
Results
Trial characteristics
Out of 29,145 abstracts screened by the initial search, 28,044 were excluded after abstract screening. Following a full-text review of 1,101 studies, 601 were excluded based on our inclusion and exclusion criteria. Ultimately, 500 trials met our criteria for the initial research question, of which 29 trials conducted in Europe, North America, Asia, and Latin America were retained in the final analysis of this specific research question. See the PRISMA flow diagram for more details about reasons for exclusion (Additional file: PRISMA flow chart).
In total, 12,538 participants provided data for our predefined meta-analyses. All participants were adults (≥ 18 years) and all trials included older adults (either ≥ 60 or ≥ 65 years) except for four trials [28,29,30,31] while five trials exclusively included immunocompromised participants [32,33,34,35,36]. None of the trials included pregnant women. One trial exclusively included healthy older adults (≥ 60 years) [37]. Most trials assessed a third dose heterologous booster vaccine compared with a third dose homologous booster vaccine [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] while four trials compared a fourth heterologous booster with a fourth homologous booster [47, 54,55,56]. The included heterologous booster vaccines encompassed viral-vectored, mRNA, protein subunit, or inactivated virus platforms (Table 2: Trials’ characteristics). Follow-up of participants varied from 7 to 365 days after randomisation for all outcomes. Inoculation intervals between the 2nd and 3rd dose, when reported, ranged from 8 to 43 weeks and 28 to 37 weeks between the 3rd dose and 4th dose (Table 2: Trials’ characteristics).
Primary outcomes
All-cause mortality
The 11 trials (N = 5883) which reported on all-cause mortality observed one death in an immunocompromised participant in the heterologous group because of a SAE (myocardial infarction) (Fig. 1). Five trials (45%) were assessed as having some concerns regarding bias (Additional file: FigS 24) and 5 trials (45%) followed participants 90 days or more (Additional file: FigS 20).
The meta-analysis suggested that the heterologous booster vaccines may have no effect on reducing all-cause mortality compared with homologous booster vaccines (RR 0.86; 95% CI 0.33 to 2.26; I2 0.0%; very low certainty evidence), with comparable fixed-model and Peto OR effect estimates (Additional file: Table S3).
The trial sequential analysis (Additional file: FigS1) showed that the cumulative Z-curve did not cross the conventional boundaries after inclusion of eleven trials, nor reached the futility boundaries, indicating a need for more trials. It is very uncertain that subgroup analyses across heterologous booster vaccine platforms (Additional file: FigS12), number of doses (Additional file: FigS17), follow-up time (Additional file: FigS20), risk of bias (Additional file: FigS24), health status (Additional file: FigS27), and trials published before and in 2023 (Additional file: FigS31) have no effect in reducing all-cause mortality.
Laboratory-confirmed symptomatic COVID-19
All trials either used reverse transcription polymerase chain reaction (RT-PCR) or similar laboratory tests for COVID-19 exclusively for those reporting symptoms. Thus, we were only able to report on symptomatic participants of COVID-19 and not all participants with confirmed COVID-19 as stated in our protocol. Fourteen trials (N = 5677) reported on symptomatic COVID-19 with 13 trials (Fig. 2) assessed as having some concerns for Domain 4 (measurement of the outcome) and one being downgraded to high risk of bias due to three domains being attributed some concerns. Seven trials (50%) followed participants 90 days or more (Additional file: FigS21). The pooled RR suggested that the heterologous booster vaccines may not have effect on risk of confirmed symptomatic COVID-19 compared with homologous booster vaccines (RR 0.95; 95% CI 0.72 to 1.25; I2 0.0%; very low certainty evidence), which was further supported by estimates from the fixed-effect model and the Peto OR (Additional file: Table S3). The TSA showed that the cumulative Z-curve did not cross the conventional boundaries after inclusion of the fourteen trials, nor reached the futility boundaries, indicating a need for more trials (Additional file: FigS2).
As authors did not report the methodology of how symptomatic COVID-19 participants were diagnosed, this was reflected by assigning some concerns in Domain 4 (measurement of the outcome), therefore precluding us from performing a subgroup analysis by risk of bias. It is uncertain that subgroup analyses according to heterologous booster vaccine platforms (Additional file: Fig S13), variations in follow-up duration (Additional file: Fig S21), health status (Additional file: Fig S28), by pre-2023 and in 2023 (Additional file: Fig S32), and according to vaccine booster valency (Additional file: Fig S34), have no effect in reducing laboratory-confirmed symptomatic COVID-19 events between the two intervention groups.
Laboratory-confirmed severe COVID-19
Ten trials (N = 4494) assessed severe disease associated with laboratory-confirmed COVID-19 (Fig. 3), with all trials having some concerns for Domain 4 (measurement of the outcome). Only two participants with severe COVID-19 were reported, which occurred in the homologous booster group. Six trials (60%) followed participants 90 days or more (Additional file: Fig S22).
The pooled random-effects model estimates that heterologous booster doses may have no effect on reducing severe COVID-19 symptoms versus homologous booster doses (RR 0.51; 95% CI 0.20 to 1.33; I2 0.0%; very low certainty), with comparable estimates from the fixed-effect model and Peto OR (Additional file: Table S3). The TSA underscored that the required meta-analytic sample size has not been met, thereby preventing the establishment of conclusive evidence (Additional file: FigS3). Therefore, additional trials are imperative to substantiate the impact of a heterologous vaccine regimen on laboratory-confirmed severe COVID-19 participants.
As trial authors did not report the methodology of how severe COVID participants were diagnosed, all trials measuring this outcome were assessed as having some concerns for Domain 4 (measurement of the outcome), therefore precluding us from performing a subgroup analysis by risk of bias. It is very uncertain that subgroup analyses across heterologous booster vaccine platforms (Additional file: FigS14), variations in follow-up duration (Additional file: FigS22), pre-2023 and in 2023 (Additional file: FigS33), and according to vaccine booster valency (Additional file: FigS35) have any effect in reducing laboratory-confirmed severe COVID-19 between the subgroups.
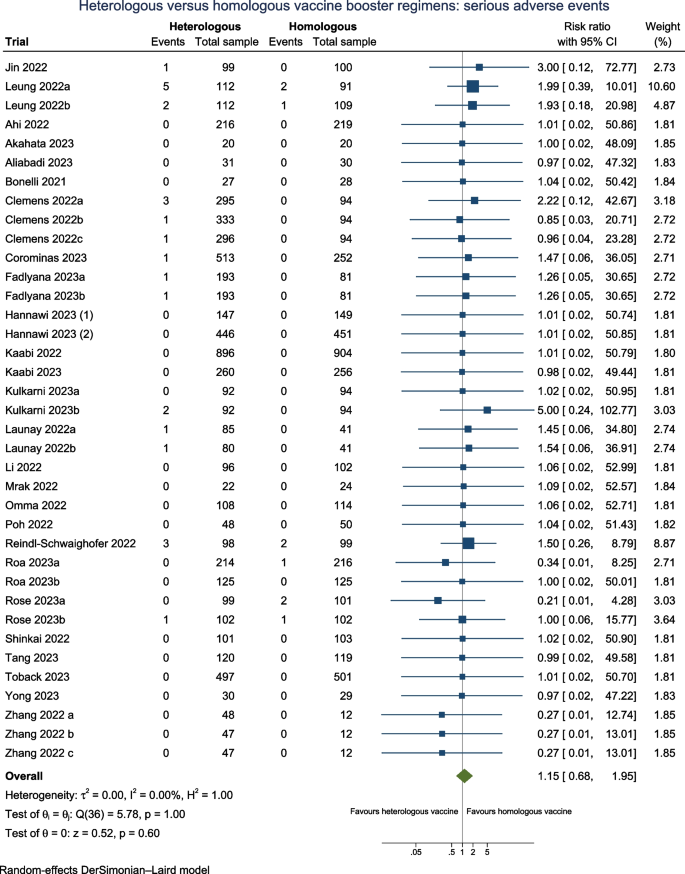
Serious adverse events
Twenty-seven trials (N = 11,384) reported serious adverse events (SAE) when assessing the safety profile of the heterologous versus homologous booster vaccines (Fig. 4), of which 13 of trials (48%) were assessed as having one or more concerns across domains of which three trials at high risk of bias. Fourteen trials (52%) followed participants 90 days or longer.
The overall estimates suggest that there may be no difference on the risk for serious adverse events between heterologous booster vaccines versus homologous booster vaccines (RR 1.15; 95% CI 0.68 to 1.95; I2 0.0%; very low certainty evidence), with comparable estimates from the fixed-effect model and Peto OR (Additional file: Table S3). The TSA reveals that the cumulative number of participants remains suboptimal, indicating the insufficiency of the accrued sample size (Additional file: FigS4). Therefore, additional trials are necessary to ascertain the impact of a heterologous vaccine regimen on serious adverse events. It is very uncertain that subgroup analyses across heterologous booster vaccine platforms (Additional file: FigS15), different doses (Additional file: FigS18), variations in follow-up duration (Additional file: FigS23), risk of bias (Additional file: FigS25), health status (Additional file: FigS29), and according to vaccine booster valency (Additional file: FigS36) may have any effect on SAE between the subgroups.
Secondary outcomes
Quality of life
None of the included trials reported on health-related QoL.
Adverse events considered not serious
Twenty trials (N = 10,008) reported on AE considered non-serious when assessing the safety profile for booster vaccines (Fig. 5), of which ten trials (50%) were considered as having one or more concerns across domains of which two were at high risks of bias. Follow-up for all trials was less than 90 days.
Most common types of AE considered non serious were fatigue, fever, injection site pain, redness, muscle pain, and headache. The overall pooled RR suggested that there may be a higher risk of AE considered non-serious by 21% in the heterologous vaccination group versus the homologous vaccination group (RR 1.19; 95% CI 1.08 to 1.32; I2 64.4%; very low certainty), with concurring estimates with the fixed-effect model and Peto OR (Additional file: Table S3). The TSA showed that the cumulative Z-curve did not intersect the threshold indicating potential harm nor potential benefit associated with heterologous vaccines after incorporating the 20 trials (Additional file: FigS5).
Subgroup analyses based on different doses (Additional file: Fig S19), risk of bias (Additional file: Fig S26), and health status (Additional file: Fig S30) did not impact the pooled relative risk (RR) or reduce heterogeneity. The lack of difference in effect due to different doses on adverse events (AEs) considered non-serious remains very uncertain across subgroups. Furthermore, the evidence for differential higher risks of non-serious AE with protein-based vaccine boosters, viral-vectored booster platforms, and mRNA vaccine booster platforms remain very uncertain due to an even higher risk of imprecision (RR 1.13; 95% CI 1.00 to 1.29; I2: 62.5%), (RR 1.51; 95% CI 1.16 to 1.97; I2: 56.2%,) and (RR 1.25; 95% CI 1.00 to 1.56), respectively (Additional file: FigS16).
Publication bias
No asymmetry for all-cause mortality, symptomatic COVID-19, severe COVID-19, and SAE (Additional file: Fig S37-40) were observed in the funnel plots, providing evidence against publication bias, which was further corroborated by Egger’s tests showing no significant evidence of publication bias. For adverse events considered non-serious, despite the presence of slight asymmetry in the funnel plot for the outcome (Additional file: FigS44), the significant result from the Egger’s test (P: 0.02) suggests evidence of publication bias for non-serious adverse events. It is noteworthy that substantial heterogeneity among the included trials could potentially account for the observed asymmetry, introducing some uncertainty into our findings.
Discussion
In this updated living vaccine project valid until the end of 2023, we focused on gathering evidence from 29 trials comparing heterologous-based booster versus homologous-based booster regimens, of which two compared multivalent versus bivalent boosters. We found no evidence of different effects on mortality, laboratory-confirmed symptomatic COVID-19, laboratory-confirmed severe COVID-19, or SAE. Our TSAs revealed that the accrued sample size was suboptimal to make any robust conclusions of any difference of effects on these outcomes. We found no data on QoL. Nevertheless, we found that heterologous booster regimens may increase the occurrence of AE considered non-serious, but more data will be required to confirm this finding.
Heterogeneity was only encountered assessing AE considered non-serious. Notably, for this outcome, subgroup analyses across vaccine platforms, doses, risk of bias, and health status of participants did not reduce the high level of heterogeneity, which remained above 50%. Due to limited sample sizes, we cannot confidently determine significant differences or lack thereof for all outcomes.
Thus, at this juncture, the very low certainty of evidence yielded from this systematic review does not allow an assessment of beneficial and harmful effects of combining the two different types of vaccine platform, thereby providing limited evidence supporting any firm conclusions. Thus, it would be premature to infer whether lack of statistical significance is due to insufficient sample size or due to no differences between heterologous and homologous booster regimens.
To our knowledge, no other systematic review comprising only randomised clinical trials exists, thus hindering direct comparisons to be made. Three meta-analyses were published between April and August 2022, with the bulk of evidence emanating from observational studies [5,6,7]. Deng et al. [6] reported higher vaccine effectiveness for symptomatic COVID-19 and severe symptoms associated with COVID-19 with heterologous boosters (56.8% compared to 17.3% and 97.4% compared to 93.4%, respectively) [6]. Conversely, Au et al. (2022) found comparable effectiveness between heterologous and homologous three-dose regimens in preventing COVID-19 symptomatic and severe infections [7]. Regarding safety outcomes, our findings align with Deng et al. [6], who reported higher odds for adverse events considered non-serious in the heterologous booster group, in disagreement with Cheng et al. [5] who reported a higher incidence of total adverse events in the homologous group booster group [5]. However, these discrepancies may be attributed to confounding factors, including location-based differences in vaccination strategies.
Strengths and limitations
Strengths related to our methodology include the use of five biomedical databases drawing from a combination of approaches to increase the likelihood of capturing all eligible trials. Second, we only included randomised clinical trials. Third, we employed our general search strategy as defined by the protocol followed by a specific search strategy tailored to our specific research question, which was later complemented with the use of the snowballing method. Fourth, we conducted TSAs to control type I and type II errors and strengthen our assessment of the imprecision domain in GRADE.
Our eligible trials have several strengths. Firstly, the inclusion of participants from diverse geographical regions supports the generalisation of results, increasing the applicability of our findings to broader populations. Furthermore, by utilising various vaccine regimen combinations in the heterologous arm, compared with different homologous vaccine regimens, we further enhance the generalisation of our results in addressing our broad research question, whether heterologous regimens are more likely to improve vaccine efficacy and safety.
However, interpretation of our findings warrants caution and cognisance of certain methodological limitations, as reflected in the very low certainty we have in the evidence, largely attributable to the non-negligible percentage of RCT not being free of potential biases, imprecision, and heterogeneity. Secondly, we were unable to adequately assess the quality of RCT reporting on vaccine efficiency as none of the eligible trials reporting on these outcomes described the methodology for assessing this efficiency. In addition, whilst including trials from different geographical regions with varying patterns of sublineage predominance, vaccination combinations, and intervals between prime and boost doses using different vaccine regimens may help generalise findings, this diversity may also lead to residual heterogeneity, as seen in the case of adverse events considered non-serious.
Whilst our study provides valuable insights into the efficacy and safety outcomes of homologous compared with heterologous vaccine regimens across various vaccine platforms, we acknowledge that the absence of trials involving recombinant protein boosters may have limited our exploration of the effect of protein-based heterologous boosters. Additionally, the majority of the trials had a follow-up time of less than 3 months, along with large inoculation time intervals between doses, potentially resulting in failure to adequately gauge benefits and harms. The absence of disaggregated data for older adults, who along with the immunocompromised population, are poised to benefit the most from a booster dose, further limits our analyses.
Hence, this systematic review underscores the imperative for more robust randomised clinical trials to corroborate either all non-significant differences observed or explore the possibility of a differential effect between heterologous versus homologous booster regimen, also among older adults.
Conclusions
Our living systematic review provides current insights into the comparative efficacy and safety of heterologous versus homologous COVID-19 booster regimens. Upon evaluating three vaccine efficacy outcomes, i.e., all-cause mortality, symptomatic COVID-19, and severe COVID-19, no adequate accrued sample size was reached to be able to conclude a lack of difference in prevention between the heterologous versus homologous booster vaccine regimens. In terms of safety outcomes, whilst heterologous vaccine regimens may lead to higher occurrences of AE considered non-serious in contrast to SAE which showed a pooled relative risk range that encompassed the line of no effect, our TSAs pointed to inadequate sample size for both outcomes. As multivalent vaccine heterologous boosters become more prominent, future randomised clinical trials should prioritise diverse populations, including older adults and immunocompromised people and ensure standardised assessment to optimise vaccination strategies and global pandemic control efforts.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse events
- CI:
-
Confidence intervals
- COVID-19:
-
Coronavirus disease 2019
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluation
- mRNA:
-
Messenger RNA
- OR:
-
Odds ratio
- QoL:
-
Quality of life
- RCT:
-
Randomised clinical trials
- RR:
-
Relative risk or risk ratio
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SAE:
-
Serious adverse events
- SARS-CoV-2:
-
Severe respiratory syndrome coronavirus 2
- TSA:
-
Trial sequential analysis
- VOIs:
-
Variants of interest
- VUMs:
-
Variants under monitoring
- WHO:
-
World Health Organization
References
WHO. COVID-19 Weekly Epidemiological Update - Edition 158. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-september-2023.
WHO. Global COVID-19 vaccination strategy in a changing world. July 2022 Update. Available from: https://www.who.int/publications/m/item/global-covid-19-vaccination-strategy-in-a-changing-world--july-2022-update.
Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57.
WHO. SAGE updates COVID-19 vaccination guidance. Available from: https://www.who.int/news/item/28-03-2023-sage-updates-covid-19-vaccination-guidance.
Cheng H, Peng Z, Si S, Alifu X, Zhou H, Chi P, et al. Immunogenicity and safety of homologous and heterologous prime–boost immunization with COVID-19 vaccine: systematic review and meta-analysis. Vaccines. 2022;10(5):798.
Deng J, Ma Y, Liu Q, Du M, Liu M, Liu J. Comparison of the effectiveness and safety of heterologous booster doses with homologous booster doses for SARS-CoV-2 vaccines: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(17):10752.
Au WY, Cheung PPH. Effectiveness of heterologous and homologous covid-19 vaccine regimens: living systematic review with network meta-analysis. BMJ. 2022;377:e069989.
Korang SK, Juul S, Nielsen EE, Feinberg J, Siddiqui F, Ong G, et al. Vaccines to prevent COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project). Syst Rev. 2020;9(1):262.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Wohlin C, Kalinowski M, Romero Felizardo K, Mendes E. Successful combination of database search and snowballing for identification of primary studies in systematic literature studies. Inf Softw Technol. 2022;147:106908.
Trial Sequential Analysis (TSA) [Computer program]. The copenhagen trial unit, centre for clinical intervention research, the capital region, Copenhagen University Hospital – Rigshospitalet. 2021. 2021.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6(3):341–8.
Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14(1):120.
Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Hebel C, Thomsen AR. A survey of mechanisms underlying current and potential COVID-19 vaccines. APMIS. 2023;131(2):37–60.
Verdecia M, Kokai-Kun JF, Kibbey M, Acharya S, Venema J, Atouf F. COVID-19 vaccine platforms: delivering on a promise? Hum Vaccines Immunother. 2021;17(9):2873–93.
Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022:e068632. https://doi.org/10.1136/bmj-2021-068632.
Hall VG, Ferreira VH, Wood H, Ierullo M, Majchrzak-Kita B, Manguiat K, et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol. 2022;23(3):380–5.
Català M, Li X, Prats C, Prieto-Alhambra D. The impact of prioritisation and dosing intervals on the effects of COVID-19 vaccination in Europe: an agent-based cohort model. Sci Rep. 2021;11(1):18812.
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75.
Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009;9(1):86.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39.
Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–9.
Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and trial sequential analysis. Syst Rev. 2018;7(1):110.
Zhang Y, Ma X, Yan G, Wu Y, Chen Y, Zhou Z, et al. Immunogenicity, durability, and safety of an mRNA and three platform-based COVID-19 vaccines as a third dose following two doses of CoronaVac in China: a randomised, double-blinded, placebo-controlled, phase 2 trial. eClinicalMedicine. 2022;54:101680.
Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28(2):401–9.
Omma A, Batirel A, Aydin M, Yilmaz Karadag F, Erden A, Kucuksahin O, et al. Safety and immunogenicity of inactive vaccines as booster doses for COVID-19 in Türkiye: a randomized trial. Hum Vaccines Immunother. 2022;18(6):2122503.
Yong X, Liu J, Zeng Y, Nie J, Cui X, Wang T, et al. Safety and immunogenicity of a heterologous booster with an RBD virus-like particle vaccine following two- or three-dose inactivated COVID-19 vaccine. Hum Vaccines Immunother. 2023;19(3):2267869.
Bonelli M, Mrak D, Tobudic S, Sieghart D, Koblischke M, Mandl P, et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann Rheum Dis. 2022;81(5):687–94.
Mrak D, Sieghart D, Simader E, Tobudic S, Radner H, Mandl P, et al. Heterologous vector versus homologous mRNA COVID-19 booster vaccination in non-seroconverted immunosuppressed patients: a randomized controlled trial. Nat Commun. 2022;13(1):5362.
Natori Y, Martin E, Mattiazzi A, Arosemena L, Ortigosa-Goggins M, Shobana S, et al. A pilot single-blinded, randomized, controlled trial comparing BNT162b2 vs. JNJ-78436735 vaccine as the third dose after two doses of BNT162b2 vaccine in solid organ transplant recipients. Transpl Int. 2023;36:10938.
Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, Jabbour R, Hofbauer TM, Merrelaar A, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182(2):165.
Sharifi Aliabadi L, Karami M, Barkhordar M, Hashemi Nazari SS, Kavousi A, Ahmadvand M, et al. Homologous versus heterologous prime-boost COVID-19 vaccination in autologous hematopoietic stem cell transplantation recipients: a blinded randomized controlled trial. Front Immunol. 2023;14:1237916.
Jin PF, Guo XL, Gou JB, Hou LH, Song ZZ, Zhu T, et al. Immunogenicity and safety of heterologous immunisation with Ad5-nCOV in healthy adults aged 60 years and older primed with an inactivated SARS-CoV-2 vaccine (CoronaVac): a phase 4, randomised, observer-blind, non-inferiority trial. Lancet Reg Health - West Pac. 2023;38: 100829.
Corominas J, Garriga C, Prenafeta A, Moros A, Cañete M, Barreiro A, et al. Safety and immunogenicity of the protein-based PHH-1V compared to BNT162b2 as a heterologous SARS-CoV-2 booster vaccine in adults vaccinated against COVID-19: a multicentre, randomised, double-blind, non-inferiority phase IIb trial. Lancet Reg Health - Eur. 2023;28:100613.
Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–76.
Kaabi NA, Yang YK, Du LF, Xu K, Shao S, Liang Y, et al. Safety and immunogenicity of a hybrid-type vaccine booster in BBIBP-CorV recipients in a randomized phase 2 trial. Nat Commun. 2022;13(1):3654.
Rose W, Raju R, Babji S, George A, Madhavan R, Leander Xavier JV, et al. Immunogenicity and safety of homologous and heterologous booster vaccination of ChAdOx1 nCoV-19 (COVISHIELDTM) and BBV152 (COVAXIN®): a non-inferiority phase 4, participant and observer-blinded, randomised study. Lancet Reg Health - Southeast Asia. 2023;100141.
Shinkai M, Sonoyama T, Kamitani A, Shibata RY, Seki NM, Omoto S, et al. Immunogenicity and safety of booster dose of S-268019-b or BNT162b2 in Japanese participants: an interim report of phase 2/3, randomized, observer-blinded, noninferiority study. Vaccine. 2022;40(32):4328–33.
Launay O, Cachanado M, Luong Nguyen LB, Ninove L, Lachâtre M, Ben Ghezala I, et al. Immunogenicity and safety of beta-adjuvanted recombinant booster vaccine. N Engl J Med. 2022;387(4):374–6.
Fadlyana E, Setiabudi D, Kartasasmita CB, Putri ND, Rezeki Hadinegoro S, Mulholland K, et al. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia. Lancet Infect Dis. 2023;23(5):545–55.
Leung NHL, Cheng SMS, Cohen CA, Martín-Sánchez M, Au NYM, Luk LLH, et al. Comparative antibody and cell-mediated immune responses, reactogenicity, and efficacy of homologous and heterologous boosting with CoronaVac and BNT162b2 (Cobovax): an open-label, randomised trial. Lancet Microbe. 2023;4(9):e670–82.
Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–9.
Roa CC, De Los Reyes MRA, Plennevaux E, Smolenov I, Hu B, Gao F, et al. Superior Boosting of Neutralizing Titers Against Omicron SARS-CoV-2 Variants by heterologous SCB-2019 vaccine vs a homologous booster in CoronaVac-primed adults. J Infect Dis. 2023;228(9):1253–62.
Ahi M, Hamidi Farahani R, Basiri P, Karimi Rahjerdi A, Sheidaei A, Gohari K, et al. Comparison of the safety and immunogenicity of FAKHRAVAC and BBIBP-CorV vaccines when administrated as booster dose: a parallel two arms, randomized, double blind clinical trial. Vaccines. 2022;10(11):1800.
Poh XY, Tan CW, Lee IR, Chavatte JM, Fong SW, Prince T, et al. Antibody response of heterologous vs homologous messenger RNA vaccine boosters against the severe acute respiratory syndrome coronavirus 2 omicron variant: interim results from the PRIBIVAC study, a randomized clinical trial. Clin Infect Dis. 2022;75(12):2088–96.
Kulkarni PS, Gunale B, Kohli S, Lalwani S, Tripathy S, Kar S, et al. A phase 3, randomized, non-inferiority study of a heterologous booster dose of SARS CoV-2 recombinant spike protein vaccine in adults. Sci Rep. 2023;13(1):16579.
Akahata W, Sekida T, Nogimori T, Ode H, Tamura T, Kono K, et al. Safety and immunogenicity of SARS-CoV-2 self-amplifying RNA vaccine expressing an anchored RBD: a randomized, observer-blind phase 1 study. Cell Rep Med. 2023;4(8): 101134.
Hannawi S, Yan L, Saf Eldin L, Abuquta A, Alamadi A, Mahmoud SA, et al. Safety and immunogenicity of multivalent SARS-CoV-2 protein vaccines: a randomized phase 3 trial. eClinicalMedicine. 2023;64:102195.
Hannawi S, Saf Eldin L, Abuquta A, Alamadi A, Mahmoud SA, Hassan A, et al. Safety and immunogenicity of a tetravalent and bivalent SARS-CoV-2 protein booster vaccine in men. Nat Commun. 2023;14(1):4043.
Toback S, Marchese AM, Warren B, Ayman S, Zarkovic S, ElTantawy I, et al. Safety and immunogenicity of the NVX-CoV2373 vaccine as a booster in adults previously vaccinated with the BBIBP-CorV vaccine: an interim analysis. Infectious Diseases (except HIV/AIDS). 2023. Available from: http://medrxiv.org/lookup/doi/10.1101/2023.03.24.23287658.
Tang R, Zheng H, Wang BS, Gou JB, Guo XL, Chen XQ, et al. Safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: a multicentre, open-label, phase 4, randomised trial. Lancet Respir Med. 2023;S2213260023000498. https://doi.org/10.1136/bmj-2021-068632.
Kaabi NA, Yang YK, Liang Y, Xu K, Zhang XF, Kang Y, et al. Safety and immunogenicity of a mosaic vaccine booster against Omicron and other SARS-CoV-2 variants: a randomized phase 2 trial. Signal Transduct Target Ther. 2023;8(1):20.
Acknowledgements
We would like to thank Sarah Klingenberg for her invaluable assistance as an information specialist at the Copenhagen Trial Unit, The Cochrane Hepato-Biliary Group, in developing and conducting the searches.
Funding
The Copenhagen Trial Unit provided support in the form of salaries for those affiliated with the centre.
Author information
Authors and Affiliations
Contributions
SM and MA conceived the specific research question and coordinated the systematic review. SKK designed the search strategy. MAA, MEM, MMB, BK, AS, and SM screened the abstracts and full texts. MAA, MEM, MMB, BK, and AS extracted the data. MAA, MEM, MMB, BK, and SM assessed the risk of bias. MAA, MEM, MMB, BK, AS, and SM were involved in the quality control of the extracted data. MAA and SM performed the data analysis. MAA, MEM, AS, and SM contributed to the first draft of the manuscript. All authors were involved in the interpretation of results and critical revision of manuscript. SM is the guarantor and attests that all authors mentioned meet authorship criteria. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SM received consulting fees as a consultant for the P-95 consulting firm. ART reports leadership or fiduciary role in other board, society, committee or advocacy group, unpaid with the Danish Society of Immunology as chairman. AS reports leadership or fiduciary role in other board, society, committee or advocacy group, unpaid with Cochrane as a steering member of the Cochrane Early Career Professionals Network. All other authors declared no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Asante, M.A., Michelsen, M.E., Balakumar, M.M. et al. Heterologous versus homologous COVID-19 booster vaccinations for adults: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMC Med 22, 263 (2024). https://doi.org/10.1186/s12916-024-03471-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03471-3