- Research article
- Open access
- Published:
A systematic review and meta-analysis of randomized trials of substituting soymilk for cow’s milk and intermediate cardiometabolic outcomes: understanding the impact of dairy alternatives in the transition to plant-based diets on cardiometabolic health
BMC Medicine volume 22, Article number: 336 (2024)
Abstract
Background
Dietary guidelines recommend a shift to plant-based diets. Fortified soymilk, a prototypical plant protein food used in the transition to plant-based diets, usually contains added sugars to match the sweetness of cow’s milk and is classified as an ultra-processed food. Whether soymilk can replace minimally processed cow’s milk without the adverse cardiometabolic effects attributed to added sugars and ultra-processed foods remains unclear. We conducted a systematic review and meta-analysis of randomized controlled trials, to assess the effect of substituting soymilk for cow’s milk and its modification by added sugars (sweetened versus unsweetened) on intermediate cardiometabolic outcomes.
Methods
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched (through June 2024) for randomized controlled trials of ≥ 3 weeks in adults. Outcomes included established markers of blood lipids, glycemic control, blood pressure, inflammation, adiposity, renal disease, uric acid, and non-alcoholic fatty liver disease. Two independent reviewers extracted data and assessed risk of bias. The certainty of evidence was assessed using GRADE (Grading of Recommendations, Assessment, Development, and Evaluation). A sub-study of lactose versus sucrose outside of a dairy-like matrix was conducted to explore the role of sweetened soymilk which followed the same methodology.
Results
Eligibility criteria were met by 17 trials (n = 504 adults with a range of health statuses), assessing the effect of a median daily dose of 500 mL of soymilk (22 g soy protein and 17.2 g or 6.9 g/250 mL added sugars) in substitution for 500 mL of cow’s milk (24 g milk protein and 24 g or 12 g/250 mL total sugars as lactose) on 19 intermediate outcomes. The substitution of soymilk for cow’s milk resulted in moderate reductions in non-HDL-C (mean difference, − 0.26 mmol/L [95% confidence interval, − 0.43 to − 0.10]), systolic blood pressure (− 8.00 mmHg [− 14.89 to − 1.11]), and diastolic blood pressure (− 4.74 mmHg [− 9.17 to − 0.31]); small important reductions in LDL-C (− 0.19 mmol/L [− 0.29 to − 0.09]) and c-reactive protein (CRP) (− 0.82 mg/L [− 1.26 to − 0.37]); and trivial increases in HDL-C (0.05 mmol/L [0.00 to 0.09]). No other outcomes showed differences. There was no meaningful effect modification by added sugars across outcomes. The certainty of evidence was high for LDL-C and non-HDL-C; moderate for systolic blood pressure, diastolic blood pressure, CRP, and HDL-C; and generally moderate-to-low for all other outcomes. We could not conduct the sub-study of the effect of lactose versus added sugars, as no eligible trials could be identified.
Conclusions
Current evidence provides a good indication that replacing cow’s milk with soymilk (including sweetened soymilk) does not adversely affect established cardiometabolic risk factors and may result in advantages for blood lipids, blood pressure, and inflammation in adults with a mix of health statuses. The classification of plant-based dairy alternatives such as soymilk as ultra-processed may be misleading as it relates to their cardiometabolic effects and may need to be reconsidered in the transition to plant-based diets.
Trial registration
ClinicalTrials.gov identifier, NCT05637866.
Background
Major dietary guidelines recommend a shift to plant-based diets for public and planetary health [1,2,3,4,5,6,7,8], while recommending simultaneous reductions in ultra-processed foods [2,3,4,5,6,7,8]. The shift to plant-based diets has resulted in an explosion of dairy, meat, and egg alternatives with plant protein foods projected to reach almost 10% of the global protein market by 2030 [9]. Although these foods can aid in the transition to plant-based diets, food classification systems such as the World Health Organization (WHO)-endorsed NOVA classification system classify them as ultra-processed foods to be avoided [10].
Dairy alternatives are an important example of a food category at the crossroads of these competing recommendations. School milk programs provide > 150 million servings of cow’s milk to children worldwide [11]. These programs are in addition to the food service and procurement policies of public institutions such as schools, universities, hospitals, long-term care homes, and prisons. Many of these programs and policies do not allow for the free replacement of cow’s milk with nutrient-dense plant milks [12, 13]. Although the Dietary Guidelines for Americans [1], Canada’s Food Guide [3], and several European food-based dietary guidelines [14] recognize fortified soymilk [1] as nutritionally equivalent to cow’s milk, school nutrition programs in the United States (US) [12] and Europe [13] only provide funding for cow’s milk. There is a bipartisan bill before the US congress to change this policy and provide funding for fortified soymilk [15]. A major barrier to the use of fortified soymilk is that it contains added sugars to match the sweetness of cow’s milk at a level which would disqualify it from meeting the Food and Drug Administration’s proposed definition of “healthy” [16] (although its total sugar content is usually ~ 60% less than that of cow’s milk given the higher sweetness intensity of sucrose vs lactose) [17] and is classified (irrespective of its sugar content) as an ultra-processed food to be avoided [10, 18]. Cow’s milk, on the other hand, enjoys classification as a “healthy,” minimally processed food to be encouraged [10, 18].
As industry innovates in response to the growing demand and policy makers develop public health nutrition policies and programs in response to the evolving dietary guidance for more plant-based diets, it is important to understand whether nutrient-dense ultra-processed plant protein foods can replace minimally processed dairy foods without the adverse cardiometabolic effects attributed to added sugars and ultra-processed foods. We conducted a systematic review and meta-analysis of randomized controlled trials of the effect of substituting soymilk for minimally processed cow’s milk and its modification by added sugars (sweetened versus unsweetened) on intermediate cardiometabolic outcomes as a basis for understanding the role of nutrient-dense ultra-processed plant protein foods in the transition to plant-based diets.
Methods
Design
We followed the Cochrane Handbook for Systematic Reviews of Interventions to conduct this systematic review and meta-analysis and reported our results by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [19, 20] (Additional file 1: Table 1). To explore whether added sugars mediate any effects observed in sweetened soymilk studies, we conducted an additional systematic review and meta-analysis sub-study. This separate investigation followed the same protocol and methodology as our main study. It focused on controlled trials examining the impact of lactose in isocaloric comparisons with fructose-containing sugars (such as sucrose, high-fructose corn syrup [HFCS], or fructose) when not included in a dairy-like matrix, on all outcomes in the main study. The protocol is registered at ClinicalTrials.gov (NCT05637866).
Data sources and search strategy
We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials databases through June 2024. The detailed search strategies for the main study and sub-study were based on validated search terms [21] (Additional file 1: Tables 2 and 4). Manual searches of the reference lists of included studies supplemented the systematic search.
Study selection
The main study included randomized controlled trials in human adults with any health status. Included trials had a study duration of ≥ 3 weeks and investigated the effects of soymilk compared with cow’s milk in energy matched conditions on intermediate cardiometabolic outcomes (Additional file 1: Table 3). Trials that included other comparators that were not cow’s milk or had no viable outcome data were excluded. No restrictions were placed on language. For the sub-study, we included controlled trials involving adults of all health statuses that had a study duration of ≥ 3 weeks and investigated the effects of added sugars compared with lactose on the same intermediate cardiometabolic outcomes (Additional file 1: Table 5).
Data extraction
A minimum of two investigators (ME, DG, SBM, AA) independently extracted relevant data from eligible studies. Extracted data included study design, sample size, sample characteristics (age, body mass index [BMI], sex, health status), intervention characteristics (soymilk volume, total sugars content, soy protein dose), control characteristics (cow’s milk volume, total sugars content, milk protein dose, milk fat content), baseline outcome levels, background diet, follow-up duration, setting, funding sources, and outcome data. The authors were contacted for missing outcome data when it was indicated that a relevant outcome was measured but not reported. Graphically presented data were extracted from figures using Plot Digitizer [22].
Outcomes
Outcomes for the main study and sub-study included blood lipids (low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], non-high-density lipoprotein cholesterol [non-HDL-C], triglycerides, and apolipoprotein B [ApoB]), glycemic control (hemoglobin A1c [HbA1c], fasting plasma glucose, 2-h postprandial glucose, fasting insulin, and plasma glucose area under the curve [PG-AUC]), blood pressure (systolic blood pressure and diastolic blood pressure), inflammation (c-reactive protein [CRP]), adiposity (body weight, BMI, body fat, and waist circumference), kidney function and structure (creatinine, creatinine clearance, glomerular filtration rate [GFR], estimated glomerular filtration rate [eGFR], albuminuria, and albumin-creatinine ratio [ACR]), uric acid, and non-alcoholic fatty liver disease (NAFLD) (intrahepatocellular lipid [IHCL], alanine transaminase [ALT], aspartate aminotransferase [AST], and fatty liver index).
Mean differences (MDs) between the intervention and control arm and respective standard errors were extracted for each trial. If these were not provided, they were derived from available data using published formulas [19]. Mean pairwise difference in change-from-baseline values were preferred over end values. When median data was provided, they were converted to mean data with corresponding variances using methods developed by McGrath et al. [23]. When no variance data was available, the standard deviation of the MDs was borrowed from a trial similar in size, participants, and nature of intervention. All disagreements were reconciled by consensus or with a senior reviewer (JLS).
Risk of bias assessment
Included studies were assessed for the risk of bias independently and in duplicate by at least two investigators (ME, DG, SBM, AA) using the Cochrane Risk of Bias (ROB) 2 Tool [24]. The assessment was performed across six domains of bias (randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias). Crossover studies were assessed for an additional domain of bias (risk of bias arising from period or carryover effects). The ROB for each domain was assessed as “low” (plausible bias unlikely to seriously alter the results), “high” (plausible bias that seriously weakens confidence in results), or “some concern” (plausible bias that raises some doubt about the results). Reviewer discrepancies were resolved by consensus or arbitration by a senior investigator (JLS).
Statistical analysis
STATA (version 17; StataCorp LP, College Station, TX) was used for all analyses for the main study and sub-study. The principal effect measures were the mean pair-wise differences in change from baseline (or alternatively, end differences) between the intervention arm providing the soymilk and the cow’s milk comparator/control arm in each trial (significance at PMD < 0.05). Results are reported as MDs with 95% confidence intervals (95% CI). As one of our primary research questions relates to the role of added sugars as a mediator in any observed differences between soymilk and cow’s milk, we stratified results by the presence of added sugars in the soymilk (sweetened versus unsweetened) and assessed effect modification by this variable on pooled estimates. Data were pooled using the generic inverse variance method with DerSimonian and Laird random effect models [25]. Fixed effects were used when less than five trials were available for an outcome [26]. A paired analysis was applied for crossover designs and for within-individual correlation coefficient between treatment of 0.5 as described by Elbourne et al. [27, 28].
Heterogeneity was assessed using the Cochran’s Q statistic and quantified using the I2 statistic, where I2 ≥ 50% and PQ < 0.10 were used as evidence of substantial heterogeneity [19]. Potential sources of heterogeneity were explored using sensitivity analyses. Sensitivity analyses were done via two methods. We conducted an influence analysis by systematically removing one trial at a time and recalculating the overall effect estimate and heterogeneity. A trial was considered influential if its removal explained the substantial heterogeneity or altered the direction, magnitude, or significance of the summary estimate. To determine whether the overall summary estimates were robust to the use of an assumed correlation coefficient for crossover trials, we conducted a second sensitivity analysis by using correlation coefficients of 0.25 and 0.75. If ≥ 10 trials were available, meta-regression analyses were used to assess the significance of each subgroup categorically and when possible, continuously (significance at P < 0.05). A priori subgroup analyses included soy protein dose, follow-up duration, baseline outcome levels, comparator, design, age, health status, funding, and risk of bias.
If ≥ 6 trials are available [29], dose–response analyses were performed using meta-regression to assess linear (by generalized least squares trend (GLST) estimation models) and non-linear spline curve modeling (by MKSPLINE procedure) dose–response gradients (significance at P < 0.05).
If ≥ 10 studies were available, publication bias was assessed by inspection of contour-enhanced funnel plots and formal testing with Egger’s and Begg’s tests (significance at P < 0.10) [30,31,32]. If evidence of publication bias was suspected, the Duval and Tweedie trim-and-fill method was performed to adjust for funnel plot asymmetry by imputing missing study data and assess for small-study effects [33].
Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the certainty of evidence. The GRADE Handbook and GRADEpro V.3.2 software were used [34, 35]. A minimum of two investigators (ME, DG, SBM) independently performed GRADE assessments for each outcome [36]. Discrepancies were resolved by consensus or arbitration by the senior author (JLS). The overall certainty of evidence was graded as either high, moderate, low, or very low. Randomized trials are initially graded as high by default and then downgraded or upgraded based on prespecified criteria. Reasons for downgrading the evidence included study limitations (risk of bias assessed by the Cochrane ROB Tool), inconsistency of results (substantial unexplained interstudy heterogeneity, I2 > 50% and PQ < 0.10), indirectness of evidence (presence of factors that limit the generalizability of the results), imprecision (the 95% CI for effect estimates overlap with the MID for benefit or harm), and publication bias (evidence of small-study effects). The evidence was upgraded if a significant dose–response gradient was detected. We defined the importance of the magnitude of the pooled effect estimates using prespecified MIDs (Additional file 1: Table 6) with GRADE guidance [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] according to five levels: very large (≥ 10 MID); large (≥ 5 MID); moderate (≥ 2 MID); small important (≥ 1 MID); and trivial/unimportant (< 1 MID) effects.
Results
Search results
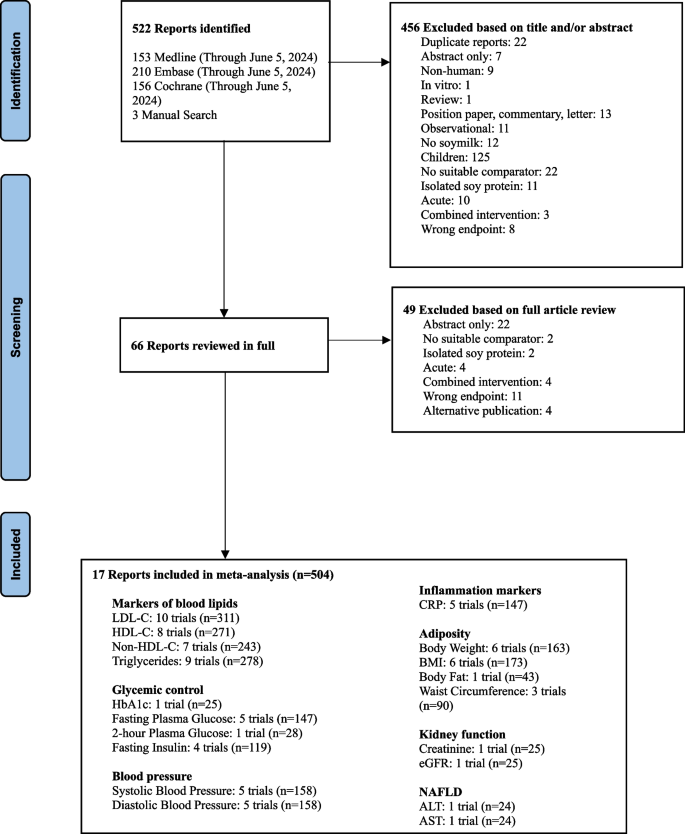
Figure 1 in Appendix shows the flow of the literature for the main analysis. We identified 522 reports through database and manual searches. A total of 17 reports [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] met the inclusion criteria and contained data for LDL (10 trials, n = 312), HDL-C (8 trials, n = 271), non-HDL-C (7 trials, n = 243), triglycerides (9 trials, n = 278), HbA1c (1 trial, n = 25), fasting plasma glucose (5 trials, n = 147), 2-h plasma glucose (1 trial, n = 28), fasting insulin (4 trials, n = 119), systolic blood pressure (5 trials, n = 158), diastolic blood pressure (5 trials, n = 158), CRP (5 trials, n = 147), body weight (6 trials, n = 163), BMI (6 trials, n = 173), body fat (1 trial, n = 43), waist circumference (3 trials, n = 90), creatinine (1 trial, n = 25), eGFR (1 trial, n = 25), ALT (1 trial, n = 24), and AST (1 trial, n = 24) involving 504 participants. No trials were available for ApoB, PG-AUC, creatinine clearance, eGFR, albuminuria, ACR, uric acid, IHCL, or fatty liver index.
Additional file 1: Fig. 1 shows the flow of literature for the sub-study. We identified 1010 reports through database and manual searches. After excluding 305 duplicates, a total of 705 reports were reviewed by title and abstract. No reports met the inclusion criteria and therefore no data was available for analysis.
Trial characteristics
Table 1 shows the characteristics of the included trials. The trials were conducted in a variety of locations, with most conducted in Iran (7/17 trials, 41%), followed by the US (3/17 trials, 18%), Italy (2/17 trials, 12%), Brazil (1/17 trials, 6%), Scotland (1/17 trials, 6%), Sweden (1/17 trials, 6%), Spain (1/17 trials, 6%), and Australia (1/17 trials, 6%). All trials took place in outpatient settings (17/17, 100%). The median trial size was 25 participants (range, 7–60 participants). The median age of the participants was 48.5 years (range, 20–70 years) and the median BMI was 27.9 kg/m2 (range, 20–31.1 kg/m2). The trials included participants with hypercholesterolemia (4/17 trials, 25%), overweight or obesity (4/17 trials, 25%), type 2 diabetes (2/17 trials, 12%), hypertension (1/17 trials, 6%), rheumatoid arthritis (1/17 trials, 6%), or were healthy (3/17 trials, 18%) or post-menopausal (2/17 trials, 12%). Both trials with crossover design (10/17 trials, 59%) and parallel design (7/17 trials, 41%) were included. The intervention included sweetened (11/17 trials, 65%) and unsweetened (6/17 trials, 35%) soymilk.
The median soymilk dose was 500 mL/day (range, 240–1000 mL/day) with a median soy protein of 22 g/day (range, 2.5–70 g/day) or 6.6 g/250 mL (range, 2.6–35 g/250 mL) and median total (added) sugars of 17.2 g/day (range, 4.0–32 g/day) or 6.9 g/250 mL (range, 1–16 g/250 mL) in the sweetened soymilk. The comparators included skim (0% milk fat) (2/17 trials, 12%), low-fat (1% milk fat) (4/17 trials, 24%), reduced fat (1.5–2.5% milk fat) (7/17 trials, 41%), and whole (3% milk fat) (1/17 trials, 6%) cow’s milk. Three trials did not report the milk fat content of cow’s milk used. The median cow’s milk dose was 500 mL/day (range, 236–1000 mL/day) with a median milk protein of 24 g/day (range, 3.3–70 g/day) or 8.3 g/250 mL (range, 3.4–35 g/250 mL) and median total (lactose) sugars of 24 g/day (range, 11.5–49.2 g/day) or 12 g/250 mL (range, 10.8–12.8 g/250 mL). The median study duration was 4 weeks (range, 4–16 weeks). The trials received funding from industry (1/17 trials, 6%), agency (8/17 trials, 47%), both industry and agency (4/16 trials, 25%), or they did not report the funding source (4/17 trials, 24%).
Risk of bias assessment
Additional file 1: Fig. 2 shows the ROB assessments of the included trials. Two trials were assessed as having some concerns from period or carryover effects: Bricarello et al. [53] and Steele [67]. All other trials were judged as having an overall low risk of bias. There was no evidence of serious risk of bias across the included trials.
Outcomes
Markers of blood lipids
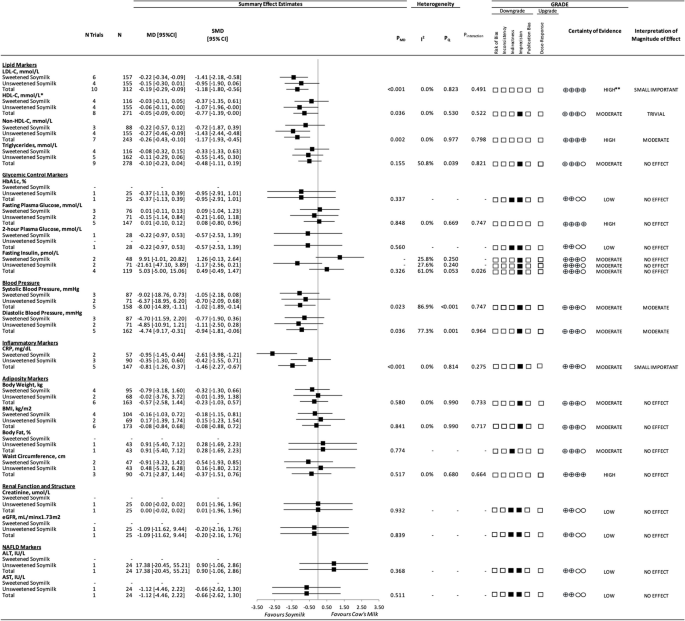
Figure 2 and Additional file 1: Figs. 3–6 show the effect of substituting soymilk for cow’s milk on markers of blood lipids. The substitution resulted in a small important reduction in LDL-C (10 trials; MD: − 0.19 mmol/L; 95% CI: − 0.29 to − 0.09 mmol/L; PMD < 0.001; no heterogeneity: I2 = 0.0%; PQ = 0.823), a trivial increase in HDL-C (8 trials; MD: 0.05 mmol/L; 95% CI: 0.00 to 0.09 mmol/L; PMD = 0.036; no heterogeneity: I2 = 0.0%; PQ = 0.053), a moderate reduction in non-HDL-C (7 trials; MD: − 0.26 mmol/L; 95% CI: − 0.43 to − 0.10 mmol/L; PMD = 0.002; no heterogeneity: I2 = 0.0%; PQ = 0.977), and no effect on triglycerides. There were no interactions by added sugars in soymilk for any blood lipid markers (P = 0.49–0.821).
Markers of glycemic control
Figure 2 and Additional file 1: Figs. 7–10 show the effect of substituting soymilk for cow’s milk on markers of glycemic control. The substitution had no effect on HbA1c, fasting plasma glucose, 2-h plasma glucose, or fasting insulin. There was no interaction by added sugars in soymilk for fasting plasma glucose (P = 0.747) but there was an interaction for fasting insulin (P = 0.026), where a lack of effect remained in both groups with neither the sweetened soymilk (non-significant increasing effect) nor the unsweetened soymilk (non-significant decreasing effect) showing an effect on fasting insulin. We could not assess this interaction for HbA1c or 2-h plasma glucose, as there was only one trial available for each outcome.
Blood pressure
Figure 2 and Additional file 1: Figs. 11 and 12 show the effect of substituting soymilk for cow’s milk on blood pressure. The substitution resulted in a moderate reduction in both systolic blood pressure (5 trials; MD: − 8.00 mmHg; 95% CI: − 14.89 to − 1.11 mmHg; PMD = 0.023; substantial heterogeneity: I2 = 86.89%; PQ ≤ 0.001) and diastolic blood pressure (5 trials; MD: − 4.74 mmHg; 95% CI: − 9.17 to − 0.31 mmHg; PMD = 0.036; substantial heterogeneity: I2 = 77.3%; PQ = 0.001). There were no interactions by added sugars in soymilk for blood pressure (P = 0.747 and 0.964).
Markers of inflammation
Figure 2 and Additional file 1: Fig. 13 show the effect of substituting soymilk for cow’s milk on markers of inflammation. The substitution resulted in a small important reduction in CRP (5 trials; MD: − 0.81 mg/dL; 95% CI: − 1.26 to − 0.37 mg/dL; PMD = < 0.001; no heterogeneity: I2 = 0.0%; PQ = 0.814). There was no interaction by added sugars in soymilk for CRP (P = 0.275).
Markers of adiposity
Figure 2 and Additional file 1: Figs. 14–17 show the effect of substituting soymilk for cow’s milk on markers of adiposity. The substitution had no effect on body weight, BMI, body fat, or waist circumference. There were no interactions by added sugars in soymilk for any adiposity outcome (P = 0.664–0.733).
Markers of kidney function
Figure 2 and Additional file 1: Figs. 18 and 19 show the effect of substituting soymilk for cow’s milk on markers of kidney function. The substitution had no effect on creatinine or eGFR. We could not assess the interaction by added sugars in soymilk for creatinine or eGFR, as there was only one trial available for each outcome which included soymilk without added sugars.
Markers of NAFLD
Figure 2 and Additional file 1: Figs. 20 and 21 show the effect of substituting soymilk for cow’s milk on markers of NAFLD. The substitution had no effect on ALT or AST. We could not assess heterogeneity or the interaction by added sugars in soymilk for ALT or AST, as there was only one trial available for each outcome which included soymilk without added sugars.
Sensitivity analysis
Additional file 1: Figs. 22–33 present the influence analyses across all outcomes. The removal of Bricarello et al. [53] or Steele [67] each resulted in loss of significant effect for HDL-C. The removal of Onning et al. [62] or Steele [67] each resulted in a partial explanation of heterogeneity for triglycerides. The removal of Hasanpour et al. [56] explained the heterogeneity for fasting insulin. The removal of Keshavarz et al. [57] or Miraghajani et al. [59] each resulted in a loss of significant effect for systolic blood pressure and the removal of Rivas et al. [63] resulted in a partial explanation of the heterogeneity for systolic blood pressure. The removal of Hasanpour et al. [56], Keshavarz et al. [57], Miraghajani et al. [59], or Rivas et al. [63] each resulted in a loss of significant effect for diastolic blood pressure and the removal of Rivas et al. [63] resulted in a partial explanation of heterogeneity for diastolic blood pressure. The removal of Mohammad-Shahi et al. [58] resulted in loss of significant effect for CRP.
Additional file 1: Table 8 shows the sensitivity analyses for the different correlation coefficients (0.25 and 0.75) used in paired analyses of crossover trials for all outcomes. The different correlation coefficients did not alter the direction, magnitude, or significance of the effect or evidence for heterogeneity, with the following exceptions: loss of significance for the effect of the substitution on HDL-C (8 trials; MD: 0.04 mmol/L; 95% CI: − 0.10 to 0.01 mmol/L; PMD = 0.107; I2 = 0.0%; PQ = 0.670) with the use of 0.25 and (8 trials; MD: 0.05 mmol/L; 95% CI: − 0.10 to 0.01 mmol/L; PMD = 0.089; I2 = 0.0%; PQ = 0.640) with the use of 0.75.
Subgroup analyses
Additional file 1: Figs. 34–36 present the subgroup analyses and continuous meta-regression analyses for LDL-C. Subgroup analysis was not conducted for any other outcome as there were < 10 trials included. There was no significant effect modification by health status, BMI, age, comparator, baseline LDL-C, study design, follow-up duration, funding source, dose of soy protein, or risk of bias for LDL-C. However, there were tendencies towards a greater reduction in LDL-C by point estimates in groups with certain health statuses (hypercholesterolemic and overweight/obesity), a higher baseline LDL-C, and a higher soy protein dose (> 25 g/day).
Dose–response analyses
Additional file 1: Figs. 37–42 present linear and non-linear dose–response analyses for LDL-C, HDL-C, non-HDL-C, triglycerides, body weight, and BMI. There was no dose–response seen for the effect of substituting soymilk for cow’s milk, with the exception of a positive linear dose–response for triglycerides (Plinear = 0.038). We did not downgrade the certainty of evidence as the greater reduction in triglycerides seen at lower doses of soy protein was lost at higher doses. There were no dose–response analyses performed for the remaining outcomes because there were < 6 trials available for each.
Publication bias assessment
Additional file 1: Fig. 43 presents the contour-enhanced funnel plot for assessment of publication bias for LDL-C. There was no asymmetry at the visual inspection and no evidence (Begg’s test = 0.721, Egger’s test = 0.856) of funnel plot asymmetry for LDL-C. No other publication bias analyses could be performed as there were < 10 trials available for each.
Adverse events and acceptability
Additional file 1: Table 9 shows the reported adverse events and acceptability of study beverages. Adverse events were reported in nine trials. In one trial by Gardner et al. [55], one participant experienced a recurrence of a cancer; however, it was considered to be unrelated to the short-term consumption of the study milks. Three trials (Miraghajani et al., Hasanpour et al., and Mohammad-Shahi, et al.) [56, 58, 59] reported one to two withdrawals due to digestive difficulties related to soymilk consumption. Two trials (Sirtori et al. 1999 and 2002) [65, 66] reported one or more participants with digestive difficulties related to cow’s milk consumption. Two trials (Nourieh et al. and Keshavarz et al.) [57, 61] each reported two participant withdrawals related to digestive problems that were not specific to either study beverage. Of these, four trials indicated that most participants found the soymilk and cow’s milk acceptable and tolerable. One trial, by Onning et al. [62], incorporated a sensory evaluation of appearance, consistency, flavor, and overall impression, which showed declining scores for both types of milk over the 3-week test period.
GRADE assessment
Additional file 1: Table 10 presents the GRADE assessment. The certainty of evidence for the effect of substituting soymilk for cow’s milk was high for LDL-C, non-HDL-C, fasting plasma glucose, and waist circumference. The certainty of evidence was moderate for HDL-C, triglycerides, fasting insulin, systolic blood pressure, diastolic blood pressure, CRP, body weight, and BMI owing to a downgrade for imprecision of the pooled effect estimates and was moderate for body fat owing to a downgrade for indirectness. The certainty of evidence was low for HbA1c, 2-h plasma glucose, creatinine, eGFR, ALT, and AST owing to downgrades for indirectness and imprecision.
Discussion
We conducted a systematic review and meta-analysis of 17 trials that examined the effect of substituting soymilk (median dose of 22 g/day or 6.6 g/250 mL serving of soy protein per day and 17.2 g/day or 6.9 g/250 mL of total [added] sugars in the sweetened soymilk) for cow’s milk (median dose of 24 g/day or 8.3 g/250 mL of milk protein and 24 g/day or 12 g/250 mL of total sugars [lactose]) and its modification by added sugars (sweetened versus unsweetened soymilk) on 19 intermediate cardiometabolic outcomes over a median follow-up period of 4 weeks in adults of varying health status. The substitution of soymilk for cows’ milk led to moderate reductions in non-HDL-C (− 0.26 mmol/L or ~ − 7%) and systolic blood pressure (− 8.00 mmHg) and diastolic blood pressure (− 4.74 mmHg); small important reductions in LDL-C (− 0.19 mmol/L or ~ − 6%) and CRP (− 0.81 mg/L or ~ 22%); and a trivial increase in HDL-C (0.05 mmol/L or ~ 4%), with no adverse effects on other intermediate cardiometabolic outcomes. There was no meaningful interaction by added sugars in soymilk, with sweetened and unsweetened soymilk showing similar effects across outcomes. There was no dose–response relationship seen across the outcomes for which dose–response analyses were performed.
Findings in relation to the literature
Our findings agree with previous evidence syntheses of soy. Regulatory authorities such as the United States Food and Drug Administration (FDA) and Health Canada have conducted comprehensive evaluations of the randomized controlled trials of the effect of soy protein from different sources on total-C and LDL-C, resulting in approved health claims for soy protein (based on an intake of 25 g/day of soy protein irrespective of source) for cholesterol reduction [68] and coronary heart disease risk reduction [69]. Updated systematic reviews and meta-analyses of the 46 randomized controlled trials included in the re-evaluation of the FDA health claim [70] showed reductions in LDL-C of − 3.2% [71]. This reduction has been stable since the health claim was first approved in 1999 [72] and is smaller but consistent with our findings specifically for soymilk. No increase in HDL-C, however, was detected. Previous systematic reviews and meta-analyses of randomized controlled trials of soy protein and soy isoflavones have also shown significant but smaller reductions in systolic blood pressure (1.70 mmHg) and diastolic blood pressure (− 1.27 mmHg) [73] than was found in the current analysis. These reductions in LDL-C and blood pressure are further supported by reductions in clinical events with updated pooled analyses of prospective cohort studies showing that legumes including soy are associated with reduced incidence of total cardiovascular disease and coronary heart disease [74].
Systematic reviews and meta-analyses that specifically isolated the effect of soymilk (as a single food matrix) in its intended substitution for cow’s milk are lacking. Sohouli and coworkers [75] conducted a systematic review and meta-analysis of 18 randomized controlled trials in 665 individuals of varying health status that assessed the effect of soymilk in comparison with a mix of comparators on intermediate cardiometabolic outcomes but did not isolate its substitution with cow’s milk. This synthesis showed similar improvements in LDL-C (− 0.24 mmol/L), systolic blood pressure (− 7.38 mmHg), diastolic blood pressure (− 4.36 mmHg), and CRP (− 1.07, mg/L), while also showing reductions in waist circumference and TNF-α [75]. The substitution of legumes that includes soy for various animal protein sources and more specifically legumes/nuts (the only exposure available) for dairy in syntheses of prospective cohort studies has also shown reductions in incident total cardiovascular disease and all-cause mortality [76].
Indirect evidence from dietary patterns that contain soy foods including soymilk in substitution for different animal sources of protein including cow’s milk further supports our findings. Systematic reviews and meta-analyses of randomized trials of the Portfolio diet and vegetarian and vegan dietary patterns have shown additive reductions in LDL-C, non-HDL-C, blood pressure, and CRP when soy foods including soymilk are combined with other foods that target these same intermediate risk factors with displacement of different animal sources of protein including cow’s milk [77, 78]. These reductions have also been shown to translate to reductions in clinical events with systematic reviews and meta-analyses of prospective cohort studies showing that adherence to these dietary patterns is associated with reductions in incident coronary heart disease, total cardiovascular disease, and all-cause mortality [79,80,81].
Potential mechanisms of action
The potential mechanism mediating the effects of soy remains unclear. Specific components within the soy food matrix, including soy protein and phytochemicals like isoflavones [82], have been implicated. The well-established lipid-lowering effect of soy [72] may be attributed to the 7S globulin fraction of soy protein, which exerts its primary action by upregulating LDL-C receptors predominantly within the liver, thereby augmenting the clearance of LDL-C from circulation [82]. The isoflavone, fiber, fatty acids, and anti-nutrient components may also exert some mediation [83]. The reduction in blood pressure has been most linked to the soy isoflavones [83]. There is evidence that soy isoflavones may modulate the renin–angiotensin–aldosterone system (RAAS), with the capacity to inhibit the production of angiotensin II and aldosterone, thereby contributing to the regulation of blood pressure [73]. Another blood pressure lowering mechanism may involve the ability of soy isoflavones to enhance endothelial function by mitigating oxidative stress and inflammation, consequently promoting the release of the relaxing factor nitric oxide (NO) [73]. This potential mechanism of isoflavones may also explain the reductions seen in inflammation.
Strengths and limitations
Our evidence synthesis had several strengths. First, we completed a comprehensive and reproducible systematic search and selection process of the available literature examining the effect of substituting soymilk for cow’s milk on intermediate cardiometabolic outcomes. Second, we synthesized the totality of available evidence from a large body of randomized controlled trials, which gives the greatest protection against systematic error. Third, we included an extensive and comprehensive list of outcomes to fully capture the impact of soymilk on cardiometabolic health. Fourth, we only included randomized controlled trials that compared soymilk to cow’s milk directly, to increase the specificity of our conclusion. Finally, we included a GRADE assessment to explore the certainty of available evidence.
There were also several limitations. First, we could not conduct the sub-study of the effect of lactose versus added sugars outside of a dairy-like matrix, as no eligible trials could be identified. Although this analysis is important for isolating the effect of added sugars as a mediator of any adverse effects, we did not observe any meaningful interaction by added sugars in soymilk. Second, there was serious imprecision in the pooled estimates across many of the outcomes with the 95% confidence intervals overlapping the MID in each case, with the exception of LDL-C, non-HDL-C, fasting plasma glucose, and waist circumference. The certainty of evidence for HDL-C, triglycerides, HbA1c, fasting plasma glucose, 2-h plasma glucose, fasting insulin, systolic blood pressure, diastolic blood pressure, CRP, body weight, BMI, body fat, creatinine, eGFR, ALT, and AST was downgraded for this reason. Third, there was evidence of indirectness related to insufficient trials for HbA1c, 2-h plasma glucose, creatinine, eGFR, ALT, and AST, which limits generalizability. Each outcome with data from only 1 trial was downgraded for this reason. Another source of indirectness could be the median follow-up duration of 4 weeks (range, 4–16 weeks). This time frame may be sufficient for observing certain effects, but other outcomes may require a longer period to manifest changes. Despite acknowledging this variation in response time among different outcomes, we did not further downgrade for this aspect of indirectness. Instead, we tailored our conclusions to reflect short-to-moderate term effects. Finally, although publication bias was not suspected, we were only able to make this assessment for LDL-C, as there were < 10 trials for all other outcomes.
Considering these strengths and limitations, we assessed the certainty of evidence as high for LDL-C and non-HDL-C; moderate for systolic blood pressure, diastolic blood pressure, CRP, and HDL-C; and moderate-to-low for all outcomes where significant effects were not observed.
Implications
This work has important implications for plant protein foods in the recommended shift to more plant-based diets. Major international dietary guidelines in the US [1], Canada [3], and Europe [4,5,6] recommend fortified soymilk as the only suitable replacement for cow’s milk. Our findings support this recommendation showing soymilk including sweetened soymilk (up to 7 g added sugars per 250 mL) does not have any adverse effects compared with cow’s milk across 19 intermediate cardiometabolic outcomes with benefits for lipids, blood pressure, and inflammation. This evidence suggests that it may be misleading as it relates to their cardiometabolic effects to classify fortified soymilk as an ultra-processed food to be avoided while classifying cow’s milk as a minimally processed food to be encouraged (based on the WHO-endorsed NOVA classification system [10]). It also suggests that it may be misleading not to allow fortified soymilk that is sweetened with small amounts of sugars to be classified as “healthy” (based on the FDA’s new proposed definition that only permits this claim on products with added sugars ≤ 2.5 g or 5% daily value (DV) per 250 mL serving [16]). The proposed FDA criteria would prevent this claim on soymilk products designed to be iso-sweet analogs of cow’s milk (in which 5 g or 10% daily value [DV] of added sugars from sucrose in soymilk is equivalent to the 12 g of lactose in cow’s milk per 250 mL serving, as sucrose is 1.4 sweeter than lactose [17]). To prevent confusion, policy makers may want to exempt fortified soymilk from classification as an ultra-processed food and allow added sugars up to 10% DV for the definition of “healthy,” as has been proposed by the FDA for sodium and saturated fat in dairy products (including soy-based dairy alternatives) to account for accepted processing and preservation methods [16]. These policy considerations would balance the need to limit nutrient-poor energy-dense foods with the need to promote nutrient-dense foods like fortified soymilk in the shift to healthy plant-based diets.
Conclusions
In conclusion, the evidence provides a good indication that substituting either sweetened or unsweetened soymilk for cow’s milk in adults with varying health statuses does not have the adverse effects on intermediate cardiometabolic outcomes attributed to added sugars and ultra-processed foods in the short-to-moderate term. There appear even to be advantages with small to moderate reductions in established markers of blood lipids (LDL-C, non-HDL-C) that are in line with approved health claims for cholesterol and coronary heart disease risk reduction, as well as small to moderate reductions in blood pressure and inflammation (CRP). Sources of uncertainty include imprecision and indirectness in several of the estimates. There remains a need for more well-powered randomized controlled trials of the effect of substituting soymilk for cow’s milk on less studied intermediate cardiometabolic outcomes, especially established markers of glycemic control, kidney structure and function, and NAFLD. There is also a need for trials comparing lactose versus added sugars outside of a dairy-like matrix to understand better the role of added sugars at different levels in substitution for lactose across outcomes. In the meantime, our findings support the use of fortified soymilk with up to 7 g added sugars per 250 mL as a suitable replacement for cow’s milk and suggest that its classification as ultra-processed and/or not healthy based on small amounts of added sugars may be misleading and need to be reconsidered to facilitate the recommended transition to plant-based diets.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1: information files.
Abbreviations
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluation
- non-HDL-C:
-
Non-high-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- CRP:
-
C-reactive protein
- HDL-C:
-
High-density lipoprotein cholesterol
- WHO:
-
World Health Organization
- US:
-
United States
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- HFCS:
-
High-fructose corn syrup
- BMI:
-
Body mass index
- ApoB:
-
Apolipoprotein B
- HbA1c:
-
Hemoglobin A1c
- PG-AUC:
-
Plasma glucose area under the curve
- GFR:
-
Glomerular filtration rate
- eGFR:
-
Estimated glomerular filtration rate
- ACR:
-
Albumin-creatinine ratio
- NAFLD:
-
Non-alcoholic fatty liver disease
- IHCL:
-
Intrahepatocellular lipid
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- MD:
-
Mean difference
- ROB:
-
Risk of bias
- 95% CI:
-
95% Confidence interval
- GLST:
-
Generalized least squares trend
- FDA:
-
Food and Drug Administration
- TNF-a:
-
Tumor necrosis factor alpha
- RAAS:
-
Renin-angiotensin-aldosterone system
- NO:
-
Nitric oxide
- DV:
-
Daily value
References
Dietary guidelines for Americans, 2020–2025. 2020 [9:[Available from: www.dietaryguidelines.gov.
Canada, Health. Canada’s Food Guide. Ottawa; 2019. https://food-guide.canada.ca/en/.
Canada’s food guide Ottawa 2019 [Available from: https://food-guide.canada.ca/en/.
Blomhoff R, Andersen R, Arnesen EK, Christensen JJ, Eneroth H, Erkkola M, Gudanaviciene I, Halldórsson ÞI, Höyer-Lund A, Lemming EW. Nordic nutrition recommendations 2023: integrating environmental aspects. Nordisk Ministerråd; 2023.
García EL, Lesmes IB, Perales AD, Arribas VM, del Puy Portillo Baquedano M, Velasco AMR, Salvo UF, Romero LT, Porcel FBO, Laín SA. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on sustainable dietary and physical activity recommendations for the Spanish population. Wiley Online Library; 2023. Report No.: 2940–1399.
Brink E, van Rossum C, Postma-Smeets A, Stafleu A, Wolvers D, van Dooren C, et al. Development of healthy and sustainable food-based dietary guidelines for the Netherlands. Public Health Nutr. 2019;22(13):2419–35.
Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM, et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472–87.
Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. The lancet. 2019;393(10170):447–92.
Bartashus J, Srinivasan G. Plant-based foods poised for explosive growth. Bloomberg Intelligence. 2021.
Monteiro CA, Cannon G, Lawrence M, Costa Louzada Md, Pereira Machado P. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome: FAO; 2019. p. 48.
International Dairy Federation. The contribution of school milk programmes to the nutrition of children worldwide. Brussels: Belgium; 2020.
USDA Food and Nutrition Service. Special Milk Program [Available from: https://www.fns.usda.gov/smp/special-milk-program.
The European Parliament. European Parliament resolution of 9 May 2023 on the implementation of the school scheme [Available from: https://www.europarl.europa.eu/doceo/document/TA-9-2023-0135_EN.html.
European Commission. Summary of FBDG recommendations for milk and dairy products for the EU, Iceland, Norway, Switzerland and the United Kingdom. [Available from: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/food-based-dietary-guidelines-europe-table-7_en.
Addressing Digestive Distress in Stomachs of Our Youth (ADD SOY) Act, House of Representatives, 1st Sess.; 2023. https://troycarter.house.gov/sites/evo-subsites/troycarter.house.gov/files/evo-media-document/add-soy-act.pdf.
Food and Drug Administration. Food labeling: nutrient content claims; definition of term “healthy”. In: Department of Health and Human Services (HHS); 2022. https://www.federalregister.gov/documents/2022/09/29/2022-20975/food-labeling-nutrient-content-claims-definition-of-term-healthy.
Helstad S. Chapter 20 - corn sweeteners. In: Serna-Saldivar SO, editor. Corn. 3rd ed. Oxford: AACC International Press; 2019. p. 551–91.
Messina M, Sievenpiper JL, Williamson P, Kiel J, Erdman JW. Perspective: soy-based meat and dairy alternatives, despite classification as ultra-processed foods, deliver high-quality nutrition on par with unprocessed or minimally processed animal-based counterparts. Adv Nutr. 2022;13(3):726–38.
Higgins J, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.2. 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
BMJ Best Practice. Search strategies [Available from: https://bestpractice.bmj.com/info/toolkit/learn-ebm/study-design-search-filters/.
Rohatgi A. WebPlotDigitizer 4.6; 2022. https://automeris.io/WebPlotDigitizer/.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):2520–37.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207.
Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9.
Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within-arm correlation imputation in trials of continuous outcomes. 2013.
Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64(11):1187–97.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook. Grading of Recommendations Assessment, Development and Evaluation, Grade Working Group. 2013.
McMaster University and Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. gradepro.org.
Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66(2):140–50.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283–93.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303–10.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–302.
Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277–82.
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66(2):158–72.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6.
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15.
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173–83.
Kaminski-Hartenthaler A, Gartlehner G, Kien C, Meerpohl JJ, Langer G, Perleth M, et al. GRADE-Leitlinien: 11. Gesamtbeurteilung des Vertrauens in Effektschätzer für einen einzelnen Studienendpunkt und für alle Endpunkte. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen. 2013;107(9):638–45.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, et al. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. J Clin Epidemiol. 2016;74:19–27.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28–39.
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–35.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH, Group, Cochrane GRADEing Methods and Group, the Cochrane Statistical Methods. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence. Cochrane handbook for systematic reviews of interventions. 2019. p. 375–402.
Azadbakht L, Nurbakhsh S. Effect of soy drink replacement in a weight reducing diet on anthropometric values and blood pressure among overweight and obese female youths. Asia Pac J Clin Nutr. 2011;20(3):383–9.
Beavers KM, Serra MC, Beavers DP, Cooke MB, Willoughby DS. Soymilk supplementation does not alter plasma markers of inflammation and oxidative stress in postmenopausal women. Nutr Res. 2009;29(9):616–22.
Bricarello LP, Kasinski N, Bertolami MC, Faludi A, Pinto LA, Relvas WG, et al. Comparison between the effects of soy milk and non-fat cow milk on lipid profile and lipid peroxidation in patients with primary hypercholesterolemia. Nutrition. 2004;20(2):200–4.
Faghih S, Hedayati M, Abadi A, Kimiagar M. Comparison of the effects of cow’s milk, fortified soy milk, and calcium supplement on plasma adipocytokines in overweight and obese women. Iranian Journal of Endocrinology and Metabolism. 2009;11(6):692–8.
Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: a randomized trial. J Am Coll Nutr. 2007;26(6):669–77.
Hasanpour A, Babajafari S, Mazloomi SM, Shams M. The effects of soymilk plus probiotics supplementation on cardiovascular risk factors in patients with type 2 diabetes mellitus: a randomized clinical trial. BMC Endocr Disord. 2023;23(1):36.
Keshavarz SA, Nourieh Z, Attar MJ, Azadbakht L. Effect of soymilk consumption on waist circumference and cardiovascular risks among overweight and obese female adults. Int J Prev Med. 2012;3(11):798–805.
Mohammad-Shahi M, Mowla K, Haidari F, Zarei M, Choghakhori R. Soy milk consumption, markers of inflammation and oxidative stress in women with rheumatoid arthritis: a randomised cross-over clinical trial. Nutr Diet. 2016;73(2):139–45.
Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35(10):1981–5.
Mitchell JH, Collins AR. Effects of a soy milk supplement on plasma cholesterol levels and oxidative DNA damage in men—a pilot study. Eur J Nutr. 1999;38(3):143–8.
Nourieh Z, Keshavarz SA, Attar MJH, Azadbakht L. Effects of soy milk consumption on inflammatory markers and lipid profiles among non-menopausal overweight and obese female adults. Int J Prev Med. 2012;3:798.
Onning G, Akesson B, Oste R, Lundquist I. Effects of consumption of oat milk, soya milk, or cow’s milk on plasma lipids and antioxidative capacity in healthy subjects. Ann Nutr Metab. 1998;42(4):211–20.
Rivas M, Garay RP, Escanero JF, Cia P Jr, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr. 2002;132(7):1900–2.
Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 2006;83(5):1118–25.
Sirtori CR, Pazzucconi F, Colombo L, Battistin P, Bondioli A, Descheemaeker K. Double-blind study of the addition of high-protein soya milk v. cows’ milk to the diet of patients with severe hypercholesterolaemia and resistance to or intolerance of statins. Br J Nutr. 1999;82(2):91–6.
Sirtori CR, Bosisio R, Pazzucconi F, Bondioli A, Gatti E, Lovati MR, et al. Soy milk with a high glycitein content does not reduce low-density lipoprotein cholesterolemia in type II hypercholesterolemic patients. Ann Nutr Metab. 2002;46(2):88–92.
Steele M. Effect on serum cholesterol levels of substituting milk with a soya beverage. Aust J Nutr Diet. 1992;49(1):24–8.
Summary of Health Canada’s assessment of a health claim about soy protein and cholesterol lowering Ottawa: Health Canada; 2015 [Available from: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/summary-assessment-health-claim-about-protein-cholesterol-lowering.html.
Food and Drug Administration. Food labeling health claims; soy protein and coronary heart disease. Fed Regist. 1999;64:57699–733.
Food and Drug Administration. Food labeling health claims; soy protein and coronary heart disease. Fed Regist. 2017;82:50324–46.
Blanco Mejia S, Messina M, Li SS, Viguiliouk E, Chiavaroli L, Khan TA, et al. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J Nutr. 2019;149(6):968–81.
Jenkins DJA, Blanco Mejia S, Chiavaroli L, Viguiliouk E, Li SS, Kendall CWC, et al. Cumulative meta-analysis of the soy effect over time. J Am Heart Assoc. 2019;8(13):e012458.
Mosallanezhad Z, Mahmoodi M, Ranjbar S, Hosseini R, Clark CCT, Carson-Chahhoud K, et al. Soy intake is associated with lowering blood pressure in adults: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Complement Ther Med. 2021;59:102692.
Viguiliouk E, Glenn AJ, Nishi SK, Chiavaroli L, Seider M, Khan T, et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv Nutr. 2019;10(Suppl_4):S308–19.
Sohouli MH, Lari A, Fatahi S, Shidfar F, Găman M-A, Guimaraes NS, et al. Impact of soy milk consumption on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Journal of Functional Foods. 2021;83:104499.
Neuenschwander M, Stadelmaier J, Eble J, Grummich K, Szczerba E, Kiesswetter E, et al. Substitution of animal-based with plant-based foods on cardiometabolic health and all-cause mortality: a systematic review and meta-analysis of prospective studies. BMC Med. 2023;21(1):404.
Chiavaroli L, Nishi SK, Khan TA, Braunstein CR, Glenn AJ, Mejia SB, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61(1):43–53.
Viguiliouk E, Kendall CW, Kahleova H, Rahelic D, Salas-Salvado J, Choo VL, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1133–45.
Glenn AJ, Guasch-Ferre M, Malik VS, Kendall CWC, Manson JE, Rimm EB, et al. Portfolio diet score and risk of cardiovascular disease: findings from 3 prospective cohort studies. Circulation. 2023;148(22):1750–63.
Glenn AJ, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, Kendall CWC, et al. Relationship between a plant-based dietary portfolio and risk of cardiovascular disease: findings from the Women’s Health Initiative prospective cohort study. J Am Heart Assoc. 2021;10(16): e021515.
Lo K, Glenn AJ, Yeung S, Kendall CWC, Sievenpiper JL, Jenkins DJA, Woo J. Prospective association of the portfolio diet with all-cause and cause-specific mortality risk in the Mr. OS and Ms. OS study. Nutrients. 2021;13(12):4360. https://doi.org/10.3390/nu13124360.
Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, et al. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140(12):2302S-S2311.
Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):324. https://doi.org/10.3390/nu9040324.
Acknowledgements
Aspects of this work were presented at the following conferences: Canadian Nutrition Society (CNS), Quebec City, Canada, May 4–6, 2023; 40th International Symposium on Diabetes and Nutrition, Pula, Croatia, June 15–18, 2023; and Nutrition 2023—American Society for Nutrition (ASN), Boston, USA, July 22–25, 2023.
Authors’ Twitter handles
@Toronto_3D_Unit.
Funding
This work was supported by the United Soybean Board (the United States Department of Agriculture Soybean Checkoff Program [funding reference number, 2411–108-0101]) and the Canadian Institutes of Health Research (funding reference number, 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3D) Centre, funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the conduct of this work. ME was funded by a CIHR Canada Graduate Scholarship and Toronto 3D PhD Scholarship award. DG was funded by an Ontario Graduate Scholarship. TAK and AZ were funded by a Toronto 3D Postdoctoral Fellowship Award. LC was funded by a Toronto 3D New Investigator Award. SA-C was funded by a CIHR Canadian Graduate Scholarship. DJAJ was funded by the Government of Canada through the Canada Research Chair Endowment. None of the sponsors had any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. But one of the co-authors, Mark Messina, who was involved in all aspects of the study except data collection or analysis, is the Director of Nutrition Science and Research at the Soy Nutrition Institute Global, an organization that receives partial funding from the principal funder, the United Soybean Board (USB).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: JLS designed the research (conception, development of overall research plan, and study oversight); ME and DG acquired the data; ME, SBM, TAK, and SAC performed the data analysis; JLS, ME, DG, SBM, AA, TAK, and LC interpreted the data; JLS and ME drafted the manuscript, have primary responsibility for the final content, and take responsibility for the integrity of the data and accuracy of the data analysis; JLS, MNE, DG, SBM, TAK, LC, AZ, SAC, AA, MM, LAL, RPB, CWCK, and DJD contributed to the project conception and critical revision of the manuscript for important intellectual content and read and approved the final version of the manuscript. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
TAK reports receiving grants from Institute for the Advancement of Food and Nutrition Sciences (IAFNS, formerly ILSI North America) and National Honey Board (USDA Checkoff program). He has received honorariums from Advancement of Food and Nutrition Sciences (IAFNS), the International Food Information Council (IFIC), the Calorie Control Council (CCC), the International Sweeteners Association (ISA), and AmCham Dubai. He has received funding from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. LC has received research support from the Canadian Institutes of health Research (CIHR), Protein Industries Canada (a Government of Canada Global Innovation Clusters), The United Soybean Board (USDA soy “Checkoff” program), and the Alberta Pulse Growers Association. AZ is a part-time research associate at INQUIS Clinical Research, Ltd., a contract research organization. She has received consulting fees from Glycemic Index Foundation Inc. SA-C has received an honorarium from the International Food Information Council (IFIC) for a talk on artificial sweeteners, the gut microbiome, and the risk for diabetes. MM was employed by the Soy Nutrition Institute Global, an organization that receives funding from the United Soybean Board (USB) and from members involved in the soy industry. RPB has received industrial grants, including those matched by the Canadian government, and/or travel support or consulting fees largely related to work on brain fatty acid metabolism or nutrition from Arctic Nutrition, Bunge Ltd., Dairy Farmers of Canada, DSM, Fonterra Inc, Mead Johnson, Natures Crops International, Nestec Inc. Pharmavite, Sancero Inc., and Spore Wellness Inc. Moreover, Dr. Bazinet is on the executive of the International Society for the Study of Fatty Acids and Lipids and held a meeting on behalf of Fatty Acids and Cell Signaling, both of which rely on corporate sponsorship. Dr. Bazinet has given expert testimony in relation to supplements and the brain. DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit Council (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI), and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from Lawson Centre Nutrition Digital Series, Nutritional Fundamentals for Health (NFH)-Nutramedica, Saint Barnabas Medical Center, The University of Chicago, 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978–0-12–810510-8), and his sister, Caroline Brydson, received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. He is also a vegan. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Lantmannen, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), National Honey Board (U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS), Pulse Canada, Quaker Oats Center of Excellence, INC International Nut and Dried Fruit Council Foundation, The United Soybean Board (USDA soy “Checkoff” program), Protein Industries Canada (a Government of Canada Global Innovation Cluster), Almond Board of California, European Fruit Juice Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF among other donors), The Plant Milk Fund at the University of Toronto (a fund established by the Karuna Foundation through Vegan Grants), and The Nutrition Trialists Network Fund at the University of Toronto (a fund established by donations from the Calorie Control Council and Physicians Committee for Responsible Medicine). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Danone, Nutrartis, Soylent, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from Danone, FoodMinds LLC, Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Arab Beverages, International Sweeteners Association, Association Calorie Control Council, and Phynova. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Ingredion, and Brightseed. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves as an unpaid member of the Board of Trustees of IAFNS. He is a Director at Large of the Canadian Nutrition Society (CNS), founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. All other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3524_MOESM1_ESM.docx
Additional file 1: This file contains Additional file 1 material, including the PRISMA checklist, further details on the search process, and additional results.
Appendix
Appendix
Flow of literature on the effect of substituting soymilk for cow’s milk on intermediate cardiometabolic outcomes. Exclusion criteria: duplicate, abstract only (conference abstract), non-human (animal study), in vitro, review/position paper/commentary/letter, observational (observational study), no soymilk (intervention was not soymilk), children (participants < 18 years of age), no suitable comparator (comparator was not cow’s milk), isolated soy protein (an ISP powder was given to participants), acute (follow-up of < 3 weeks), combined intervention (effects of intervention and comparator could not be isolated), wrong endpoint (no data for outcomes of interest), alternative publication (repeated data from original publication)
A summary plot for the effect of substituting soymilk for cow’s milk on intermediate cardiometabolic outcomes. Analyses were conducted using generic, inverse variance random-effects models (at least 5 trials available), or fixed-effects models (fewer than 5 trials available). Between-study heterogeneity was assessed by the Cochrane Q statistic, where PQ < 0.100 was considered statistically significant, and quantified by the I2 statistic, where I2 ≥ 50% was considered evidence of substantial heterogeneity. The GRADE of randomized controlled trials are rated as “high” certainty of evidence and can be downgraded by 5 domains and upgraded by 1 domain. The white squares represent no downgrades, the filled black squares indicate a single downgrade or upgrades for each outcome, and the black square with a white “2” indicates a double downgrade for each outcome. Because all included trials were randomized or nonrandomized controlled trials, the certainty of the evidence was graded as high for all outcomes by default and then downgraded or upgraded based on prespecified criteria. Criteria for downgrades included risk of bias (downgraded if most trials were considered to be at high ROB); inconsistency (downgraded if there was substantial unexplained heterogeneity: I2 ≥ 50%; PQ < 0.10); indirectness (downgraded if there were factors absent or present relating to the participants, interventions, or outcomes that limited the generalizability of the results); imprecision (downgraded if the 95% CI crossed the minimally important difference (MID) for harm or benefit); and publication bias (downgraded if there was evidence of publication bias based on the funnel plot asymmetry and/or significant Egger or Begg test (P < 0.10)), with confirmation by adjustment using the trim-and-fill analysis of Duval and Tweedie. The criteria for upgrades included a significant dose–response gradient. For the interpretation of the magnitude, we used the MIDs to assess the importance of magnitude of our point estimate using the effect size categories according to the new GRADE guidance. Then, we used the MIDs to assess the importance of the magnitude of our point estimates using the effect size categories according to the GRADE guidance as follows: a large effect (≥ 5 × MID); moderate effect (≥ 2 × MID); small important effect (≥ 1 × MID); and trivial/unimportant effect (< 1 MID). *HDL-C values reversed to show benefit. **LDL-C was not downgraded for imprecision, as the degree to which the upper 95% CI crosses the MID is not clinically meaningful. Additionally, the moderate change in non-HDL-C, with high certainty of evidence, substantiates the high certainty of the LDL-C results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Erlich, M.N., Ghidanac, D., Blanco Mejia, S. et al. A systematic review and meta-analysis of randomized trials of substituting soymilk for cow’s milk and intermediate cardiometabolic outcomes: understanding the impact of dairy alternatives in the transition to plant-based diets on cardiometabolic health. BMC Med 22, 336 (2024). https://doi.org/10.1186/s12916-024-03524-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03524-7