- Review
- Open access
- Published:
Pregnancy complications and autoimmune diseases in women: systematic review and meta-analysis
BMC Medicine volume 22, Article number: 339 (2024)
Abstract
Background
Pregnancy complications might lead to the development of autoimmune diseases in women. This review aims to summarise studies evaluating the association between pregnancy complications and the development of autoimmune diseases in women.
Methods
Medline, CINAHL, and Cochrane databases were searched up to January 2024. Nineteen pregnancy complications and 15 autoimmune conditions were included. Title, abstract, full-text screening, data extraction, and quality assessment were performed by two reviewers independently. Data were synthesised using narrative and quantitative methods. Results were presented using odds ratios (OR), relative risks (RR), incidence rate ratios (IRR), and 95% confidence intervals (CI).
Results
Thirty studies were included. One study reported composite exposure to pregnancy complications had a risk of any autoimmune disease RR 3.20 (2.90–3.51) compared to women without pregnancy complications. Women with hyperemesis gravidarum had a higher risk of developing coeliac disease (n = 1) IRR 1.98 (1.27–2.94), Crohn’s disease (n = 1) IRR 1.61 (1.25–2.04), psoriasis (n = 1) IRR 1.33 (1.01–1.71), and rheumatoid arthritis (n = 2) IRR 1.35 (1.09–1.64). Miscarriage associated with subsequent diagnosis of Sjogren syndrome (n = 2) IRR 1.33 (1.06–2.81) and rheumatoid arthritis (n = 4) OR 1.11 (1.04–1.20). Gestational hypertension/preeclampsia was linked with the development of systemic sclerosis (n = 2) IRR 2.60 (1.10–4.60) and T1DM (n = 2) IRR 2.37 (2.09–2.68). Stillbirth associated with composite autoimmune conditions (n = 2) RR 5.82 (95% CI 4.87–6.81) and aIRR 1.25 (1.12–1.40). Postpartum psychosis was associated with autoimmune thyroid disease (n = 1) aIRR2.26 (1.61–2.90).
Conclusions
Women with pregnancy complications subsequently had a higher risk of being diagnosed with autoimmune conditions. Whether this is due to pre-existing undiagnosed health conditions or being causally linked to pregnancy complications is not known.
What is already known about this subject?
-
The prevalence of autoimmune conditions and pregnancy complications has increased globally.
-
Women with pregnancy complications are at higher risk of cardiometabolic conditions in later life.
What does this study add?
-
This systematic review consolidates evidence from studies which have studied the association of pregnancy complications and the later development of autoimmune diseases in women.
-
This review provides new knowledge to help establish the association of pregnancy complications and autoimmune diseases and identifies the need for further research to establish the true association between few conditions like the development of SLE or rheumatoid arthritis followed by miscarriage.
How might this impact on clinical practice?
-
This study will be useful for health professionals and policymakers to navigate the research findings and identify the need for clinical guidelines beyond postnatal care for women with pregnancy complications.
Background
The prevalence of autoimmune diseases has been increasing globally over the last decade [1], and in the UK, 1 in 10 people have an autoimmune disease [1,2,3]. The majority of autoimmune diseases are more common in women than men [4] and are a leading cause of death in women between the age of 65 and 75 in the US and UK [5, 6]. Although the aetiology of autoimmunity is still not fully understood, the increased prevalence of autoimmune disease has been linked to defective X chromosome inactivation [7, 8] and the effects of female hormones [9].
During pregnancy, there are significant fluctuations in hormone levels and increased physiological stress. Women with pre-existing autoimmune diseases may experience flare-ups or a decrease in their symptoms. For example, rheumatoid arthritis, Grave’s disease, or psoriasis may improve during pregnancy [10,11,12], whilst patients with systemic lupus erythematosus (SLE) or multiple sclerosis are at an increased risk of disease exacerbations [13, 14]. With an increasing trend in pregnancy complications due to factors such as older age at pregnancy and women entering pregnancy with pre-existing long-term health conditions [15,16,17,18,19,20,21,22,23], it is important to study the role of pregnancy complications in the development of autoimmune diseases. Whilst it is well-established that women with autoimmune diseases have an increased risk of fertility problems and adverse pregnancy outcomes such as miscarriage and foetal growth restriction [24,25,26,27,28,29,30], less is known about the risk of developing autoimmunity in women who experience pregnancy complications [31]. Some studies have shown an association between parity and increased risk of Hashimoto thyroiditis, Sjögren’s syndrome, Graves’ disease, and rheumatoid arthritis [32, 33]. Moreover, the association between gestational diabetes mellitus (GDM) and the development of type 1 diabetes (T1DM) is well established [34]. Some pregnancy complications such as hyperemesis gravidarum and gestational hypertension have been associated with the development of rheumatoid arthritis [35, 36], whilst other studies have reported that pregnancy loss and gestational hypertension are associated with the development of SLE and systemic sclerosis [37, 38]. But other studies conducted on these associations have reported contradictory/inconsistent findings [39, 40].
This systematic review aims to determine the association between a wide range of pregnancy complications and the development of autoimmune diseases in women.
Methods
This systematic review and meta-analysis have been conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Reporting Guidelines for Meta-analyses of Observational Studies (MOOSE) (Additional file 1 table 2 and Additional file 2 table 1) [41]. The protocol for this review was registered with Prospero CRD42023412549.
Inclusion and exclusion criteria
Cohort, cross-sectional, or case-control studies reporting on the associations between pregnancy complications and the risk of autoimmune diseases were included. No language restrictions were applied. The population considered were pregnant women without any age restriction. The pregnancy complications (19) and autoimmune diseases (15) selected for inclusion were those that were more common in women, after consultation with experts in the subject (obstetricians, obstetric physicians, rheumatologists, and epidemiologists), and after input from patient and public involvement and engagement (PPIE) group members.
The pregnancy complications included are listed in Table 1 and autoimmune disease in Table 2.
Search strategy
Medline, CINAHL, and Cochrane Library were searched for studies from 2010 till January 2024.
The search strategies used pre-defined keywords of the exposures (pregnancy complications) and outcomes (autoimmune disease). Terms/keywords for each of the pregnancy complications (early adj3 pregnancy loss*, mp.miscarriage.mp, GDM) and autoimmune diseases (for example arthritis, rheumatoid systemic lupus, or SLE) were used in the search strategy. Google Scholar was searched to identify other grey literature. In addition, the reference list of the included studies, systematic reviews, and scoping reviews were searched manually to minimise the possibility of missing any relevant studies. Letters, commentaries, or editorials were excluded and studies that did not involve humans were also excluded. The searches were repeated periodically to identify newly published studies. The detailed search strategy for Medline is presented in the Additional file 1 table 1 (Table 1). This search strategy was adapted for use in other databases (CINAHL and Cochrane library). Pragmatic approach was taken given there was a substantial number of studies that needed screening (n = 24,340) and the study period was limited from 2010 to 2023 (n = 13,234). But this was complimented by a secondary search strategy looking at references of included studies, systematic reviews, scoping reviews, and by discussing with topic experts (KN, FC) to minimise the possibility of missing any study before 2010.
Study selection
EndNote reference manager [42] was used for the title and abstract screening by two researchers independently (MS, JW). Full text of the eligible reviews was screened by two researchers independently (MS, FF). Covidence software [43] was used for full-text screening and data extraction. A third senior researcher was consulted to resolve any discrepancies in the selection of the studies (FC, KN).
Data extraction
Two reviewers extracted data from the included studies. The data extraction form was adapted from JBI (Joanna Briggs Institute) data extraction form [44]. A standardised data extraction form was used and was piloted before use. The data were extracted for the following fields: author/s, year of publication, geographical area, aim of the study, population, exposures, comparator, outcomes, covariates, study design, definition of exposure, risk of bias assessment tool and result, number of participants included in the study, summary estimates, authors’ conclusion, and study limitations. The data extraction form is enclosed in Additional file Table 6.
Quality assessment
The quality of included cohort, cross-sectional, and case-control studies was assessed using the Newcastle–Ottawa scale that measures study quality based on selection, comparability of the exposure and comparator groups, and the ascertainment of outcomes and exposures [45]. The scale has an overall score of 8 points for cohort or case–control studies, and 7 for cross-sectional studies with a maximum of 1 point for each numbered item within the selection and outcome/exposure categories and a maximum of 2 points for the comparability category. We defined studies with a score of ≥ 7 points as low-risk of bias studies (very good), studies with a score of 6 points as moderate-risk of bias studies (good), and those with a score of ≤ 5 points as high-risk of bias studies (satisfactory).
Data synthesis
The effect estimates were reported as adjusted incidence rate ratios (aIRR), adjusted hazards ratios (aHR), adjusted odds ratios (aOR), or adjusted relative risks (aRR) and 95% confidence intervals (CI). We converted these effect estimates using appropriate methods (where possible) to maintain uniformity across studies [46]. Where more than one study reported the same exposure and outcome, a meta-analysis was conducted using a random effects model to generate a summary estimate. Statistical heterogeneity was estimated using the I2 statistic. To deal with potentially missing data (sample size, number exposed and unexposed), Additional file 1 of each included study was checked thoroughly, and the authors of the studies were contacted to request the data. If the data was not available and a meta-analysis could not be conducted, then effect estimates were reported as they were published. Where statistical pooling was not possible, the findings were presented in a narrative form including tables and figures to aid data presentation. R (3.3.0) and R Studio (12.1) were used to conduct statistical analysis [47,48,49].
Patient and public involvement
Patient and public involvement and engagement (PPIE) representatives (RP and NM) participated in formulating the research question. They have also played key role in collaboration with clinicians and researchers to identify and consider the list of pregnancy complications and autoimmune diseases in the study. They will play a key role in disseminating the results.
Results
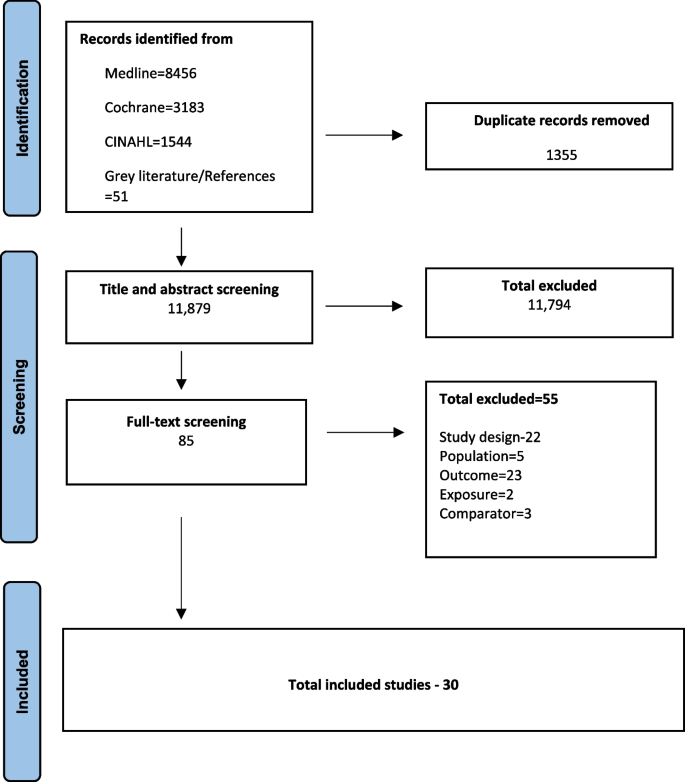
Out of the 13,234 records identified from the search and after full-text screening of 85 studies, 30 studies were included [31, 34,35,36, 39, 40, 50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. Studies were excluded if they did not qualify in the study inclusion criteria based on the study design (n = 22), population (n = 5), intervention (2), outcome (23), and comparator(3). The selection process is shown in the PRISMA diagram (Fig. 1) [41]. The list of excluded studies is provided in Additional file 1 table 3.
Characteristics of the included studies are reported in Table 3. Out of the 30 studies, the majority were prospective cohort studies (n = 21), 8 were retrospective case–control studies, and 1 was a cross-sectional study. There were 23 studies conducted in Europe, the remainder were in Taiwan (2), China (2), South Korea (1), and the United States (2). In most cohort studies (n = 14), information about the pregnancy complications and autoimmune diseases was collected through medical records. Medical records were used to establish the autoimmune diseases in case–control studies, and questionnaires were used to determine the pregnancy complications. The follow-up period of the cohort studies varied from 9 months to 26 years with a median of 12 years. Out of the 21 cohort studies, 9 used the data from the same cohort (Danish national registry) [31, 36, 39, 53, 54, 63, 64, 69]. In the instance of two studies reporting the same exposure and outcome, the most recent study was used to avoid duplication. We have done this in accordance with the Cochrane handbook for systematic reviews [74]. For instance, two studies, Mikkelsen et al. [63] and Nielsen et al. [64], were both reporting the association of pregnancy complications and the future development of multiple sclerosis in women using Danish Civil Registration System. Mikkelsen et al.’s study was used to report the findings in this review. Details have been added in Additional file tables 1 and 5 [75, 76]. A total of 18 different pregnancy complications and 12 autoimmune diseases (including an overall “all autoimmune diseases”) were investigated across the studies. Rheumatoid arthritis (8 studies) and SLE (5 studies) were two of the most included outcomes. The meta-analysis performed in this systematic review is included in Additional file 1 figure 1.1–1.11.
Quality assessment
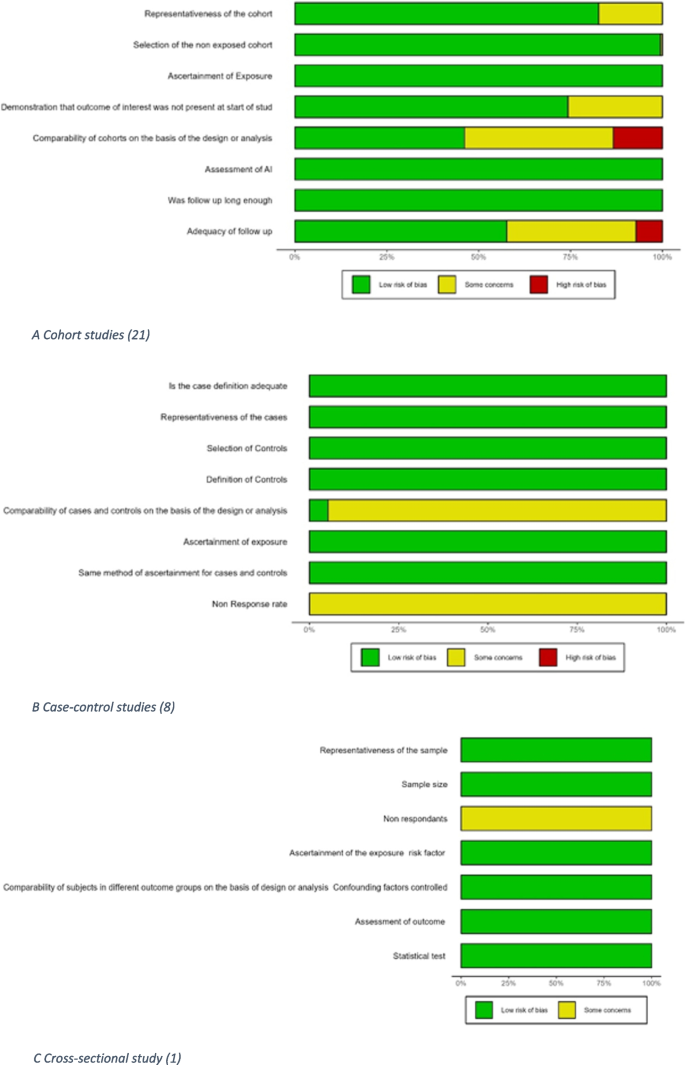
Results of the quality assessment of the studies using the Newcastle–Ottawa scale are shown in Fig. 2 and Additional file 1 Table 4.1–4.3 [77]. Eighteen out of 30 studies had a low risk of bias with an overall “very good” rating. The principal areas of concern were the comparability of cohorts and the adequacy of follow-up.
All autoimmune diseases
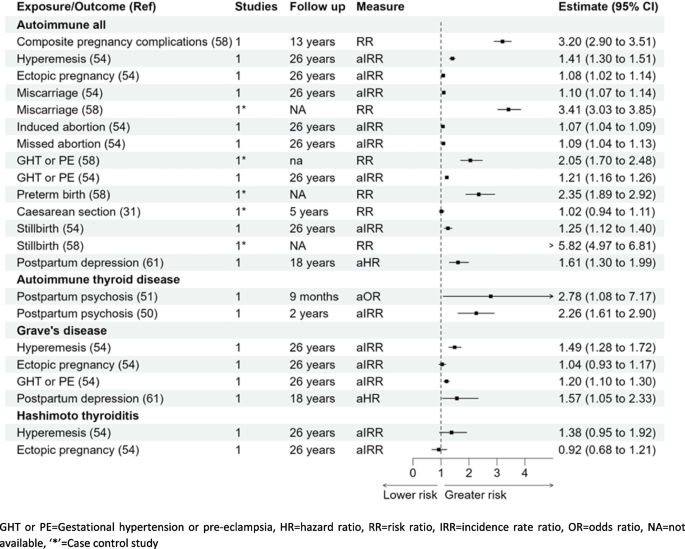
There was more than a threefold higher risk of developing autoimmune diseases (n = 7) in women with pregnancy complications (n = 6) RR 3.20 (95% CI 2.90–3.51) when compared with women without pregnancy complications [58]. Out of the pregnancy complications studied individually, two studies reported, women with previous miscarriage RR 3.41 (3.03–3.85) and aIRR 1.10 (1.07–1.14) was reported to have higher risk [58]. One cohort and one case–control study reported a higher risk of autoimmune diseases in women with gestational hypertension or pre-eclampsia; RR 2.05 (1.70–2.48) and aIRR 1.21 (1.16–1.26) [54, 58], respectively. Women with stillbirth were reported to have higher chances to have autoimmune disease in later life reported by two studies RR 5.82 (4.97–6.81) [58] and aIRR 1.25 (1.12–1.40) [54]. There was a significantly higher risk of developing autoimmune diseases for women with preterm birth RR 2.35 (1.89–2.92) [58]. There was little association reported with caesarean section, induced abortion, or postpartum depression with the development of autoimmune diseases [31, 54, 61]. However, a study reported a higher risk of developing autoimmune diseases in women with perinatal depression aHR 1.52 (1.46–1.58), with antenatal depression aHR 1.50 (1.43–1.58), and postpartum depression aHR 1.55 (1.45–1.65) [73].
Autoimmune thyroid diseases
Hyperemesis gravidarum aIRR 1.49 (1.28–1.72), gestational hypertension or pre-eclampsia aIRR 1.20 (1.10–1.30), and postpartum depression aHR 1.57 (1.05–2.33) [54, 60] were all associated with a higher risk of Grave’s disease but there was no significant association between ectopic pregnancy and Grave’s disease aIRR 1.04 (0.93–1.17) [54]. Gestational hypertension/pre-eclampsia aIRR 1.41 (1.17–1.68) was associated with a higher risk of Hashimoto’s thyroiditis but there was little association of hyperemesis gravidarum or ectopic pregnancy with Hashimoto’s thyroiditis; aIRR 1.38 (0.95–1.92) and aIRR 0.92 (0.68–1.21), respectively [54]. Two cohort studies reported a greater risk of autoimmune thyroid disease in women with postpartum psychosis aOR 2.78 (1.08–7.17) at 9 months and aIRR 2.26 (1.61–2.90) with 2 years follow-up postpartum when compared with women without postpartum psychosis [50, 51].
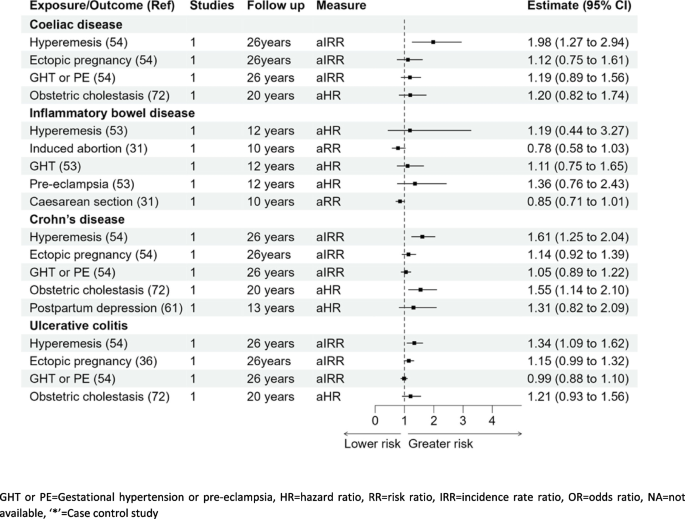
Coeliac disease
Women who experienced hyperemesis gravidarum had almost a twofold risk of coeliac disease compared to women without; aIRR 1.98 (1.27–2.94) [54]. None of the other pregnancy complications were significantly associated with coeliac disease; ectopic pregnancy aIRR 1.12 (0.75–1.61), gestational hypertension, pre-eclampsia aIRR 1.19 (0.89–1.56), and intrahepatic cholestasis of pregnancy aHR 1.20 (0.82–1.74) [54, 72].
Inflammatory bowel disease (Crohn’s disease and ulcerative colitis)
Out of the five pregnancy complications reported for IBD (hyperemesis gravidarum, missed abortion, gestational hypertension, pre-eclampsia, and Caesarean section), none of these associations were statistically significant. However, studies reporting ulcerative colitis and Crohn’s disease separately found significant associations. Hyperemesis was significantly associated with the development of both ulcerative colitis and Crohn’s disease, aIRR 1.34 (1.09–1.62) and aIRR 1.61 (1.25–2.04), respectively [54]. Furthermore, a higher risk of Crohn’s disease was also observed in women with intrahepatic cholestasis of pregnancy, HR 1.55 (1.14–2.10) [72]. No other pregnancy complications were associated with the development of IBD as reported in Fig. 4.
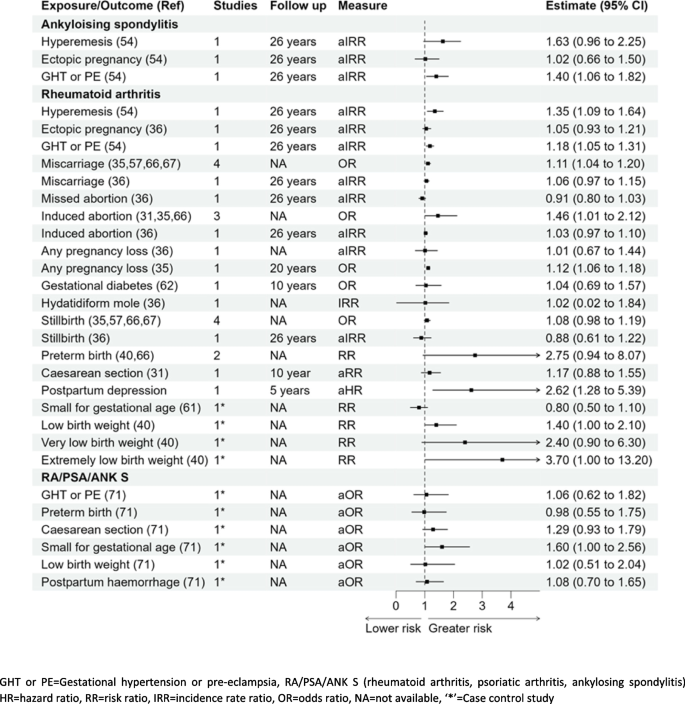
Ankylosing spondylitis
Out of the three pregnancy complications studied with the development of ankylosing spondylitis in women, there was no significant association for hyperemesis gravidarum IRR 1.63 (0.96–2.25) or ectopic pregnancy IRR 1.02 (0.66–1.50); a significant association was noted with gestational hypertension and pre-eclampsia IRR 1.40 (1.06–1.82) [54] (Fig. 5).
Rheumatoid arthritis
Out of the five studies reporting the association of miscarriage and rheumatoid arthritis, four were meta-analysed to estimate 11% higher odds: pooled OR 1.11 (1.04–1.20) with the other study showing a slightly elevated risk that was not statistically significant aIRR 1.06 (0.97–1.15) [35, 36, 54, 57, 66, 67]. A significant association was also reported with hyperemesis, gestational hypertension, and pre-eclampsia with aIRR 1.35 (1.09–1.64), aIRR 1.18 (1.05–1.31), respectively [54]. [35, 36, 54, 57, 66, 67]. Whilst three studies were pooled to derive a significant association between rheumatoid arthritis and induced abortion 1.46 OR (1.01–2.12). Women with any pregnancy loss were reported to be at higher risk of developing the disease in one study aIRR 1.12 (1.06–1.12) and others reported no association aIRR 1.01 (0.67–1.44), and this will require further research to establish the true association. The association for induced abortion or any pregnancy loss with rheumatoid arthritis reported mixed findings with significant association reported by few studies and insignificant by others as shown in Fig. 5 [31, 35, 36, 54, 57, 66, 67]. A higher risk of developing rheumatoid arthritis was observed in women who delivered “extremely low birth weight” babies (< 1000 g) with RR 3.70 (1.00–13.20) or “low birth weight” babies (< 2500 g) with RR 1.40 (1.00–2.10) when compared to women who delivered normal birth weight babies [40]. An increased risk of rheumatoid arthritis was also reported for women with postpartum depression with aHR 2.62 (1.28–5.39) [60]. No other pregnancy complications studied in relation with the development of rheumatoid arthritis were statistically significant (Fig. 5). There was also no significant association with the development of rheumatoid arthritis as reported in women who delivered very low birth weight babies (< 1500 g) in a study with a small sample size (n = 20) [40].
Rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis (composite outcome)
There were no other significant associations with rheumatoid arthritis or the composite outcome with other pregnancy complications studied (gestational hypertension or pre-eclampsia, caesarean section, postpartum haemorrhage, or mothers delivering preterm births or low birth weight babies) (Fig. 5) [71]. Women who delivered small for gestational age babies were more likely to have the composite outcome of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis aOR 1.60 (1.00–2.56) when compared to women with normal for gestational age babies.
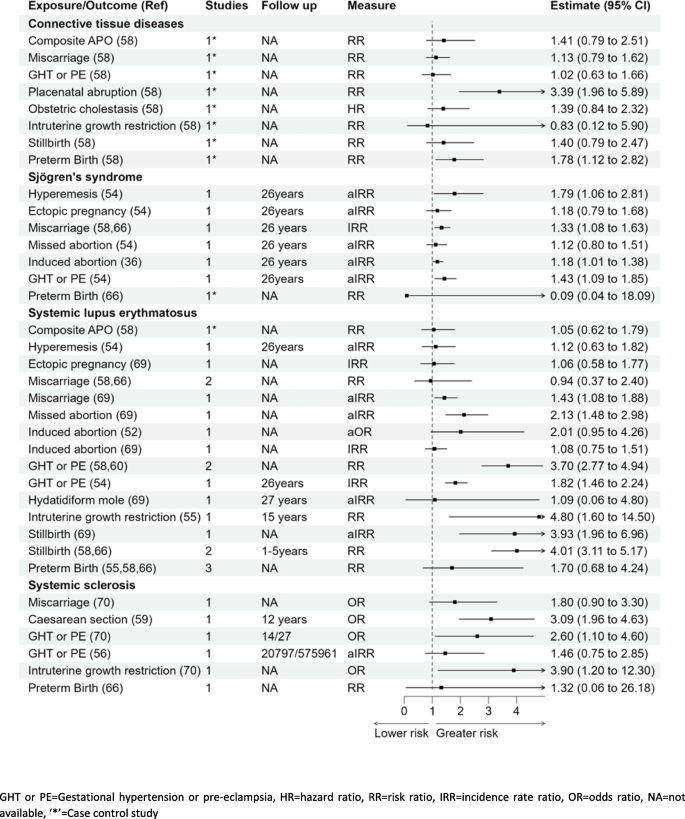
Connective tissue diseases (systemic lupus erythematosus, Sjogren syndrome, systemic sclerosis)
Connective tissue disease
Seven pregnancy complications were reported in association with connective tissue disease (CTD). Women with placental abruption or preterm birth had significantly higher risk of CTD; RR 3.39 (1.96–5.89) and RR 1.78 (1.12–2.82), respectively, [58] in Fig. 6. The associations of gestational hypertension, intrauterine growth restriction (IUGR), miscarriage, stillbirth, and composite pregnancy complications with CTD were not statistically significant [58].
Systemic lupus erythematosus (SLE)
Results for an association between miscarriage and SLE were mixed with one study reporting aIRR of 1.43 (1.08–1.88), and a pooled RR for two smaller studies showing no significant association 0.94 (0.37–2.40) [54, 58, 66, 69]. However, there was a significant association reported with any pregnancy loss and future development of the disease with OR 1.87 (1.31–2.67) [52, 55]. Missed abortions were associated with a higher risk of SLE; IRR 2.13 (1.48–2.98) [54, 69]. Women with history of IUGR had almost a fivefold higher risk of SLE; RR 4.80 (1.60–14.50) when compared with women with no IUGR [55]. Results from three studies showed that a history of stillbirth was associated with a four times higher risk of SLE, pooled RR 4.01 (3.11–5.17) and pooled IRR 3.29 (3.22–4.88) [54, 58, 66, 69].
Systemic sclerosis
There was an association between gestational hypertension or pre-eclampsia and systemic sclerosis in two studies: OR 2.60 (1.10–4.60). Kamper et al. [56] reported a significant association with the development of localised scleroderma in women with pre-eclampsia with IRR 1.69 (1.02–2.80) but a nonsignificant association with subset of systemic disease aIRR 1.46 (0.75–2.80) [56, 70]. There was a three- to fourfold higher risk of systemic sclerosis for women with IUGR compared with women with normal foetal growth OR 3.90 (1.20–12.30) [70] and caesarean birth compared to vaginal birth RR 3.09 (1.96–4.63) [59].
Sjögren’s syndrome
There were seven pregnancy complications examined in relation to Sjögren’s syndrome with results showing a greater risk with hyperemesis gravidarum, aIRR 1.79 (1.06–2.81); miscarriage, aIRR 1.33 (1.08–1.63); induced abortion, aIRR 1.18 (1.01–1.38); and gestational hypertension or pre-eclampsia, aIRR 1.43 (1.09–1.85) [54]. The associations for ectopic pregnancy, missed abortion, or preterm birth, aIRR 1.18 (0.79–1.68), aIRR 1.12 (0.80–1.51), and RR 0.09 (0.04–18.09), respectively [54, 58, 66], were not statistically significant.
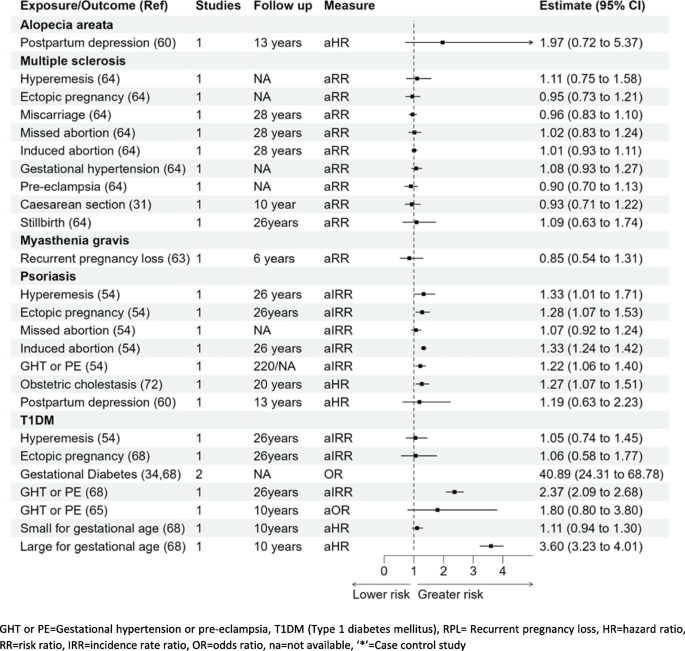
Type 1 diabetes mellitus (T1DM)
Hyperemesis gravidarum aIRR 1.05 (0.74–1.45) and ectopic pregnancy aIRR 1.06 (0.58–1.77) were not significantly associated with T1DM [34, 54, 65, 68] in Fig. 7. Results from one cohort study showed that gestational hypertension or pre-eclampsia was associated with a twofold higher risk of T1DM; aIRR 2.37 (2.09–2.68) [68], whereas in the other study, the association was higher but not statistically significant; OR 1.80 (0.80–3.80) [65]. Results from two studies showed that the risk of T1DM for women with gestational diabetes was considerably higher; pooled OR 40.89 (24.31–68.78) [34, 65]. There was almost a fourfold higher risk of T1DM in women who delivered large for gestational age babies compared to women delivering normal weight for gestational age babies aHR 3.60 (3.23–4.01) [68]. There was no significant association for women who delivered small for gestational age babies and T1DM; aHR 1.11 (0.94–1.30) [62, 71].
Psoriasis
Out of the seven pregnancy complications, five complications were associated with a higher risk of psoriasis: hyperemesis gravidarum HR 1.33 (1.01–1.71), ectopic pregnancy aIRR 1.28 (1.07–1.53), induced abortions aIRR 1.33 (1.24–1.42), gestational hypertension or pre-eclampsia aIRR 1.22 (1.06–1.40), and intrahepatic cholestasis of pregnancy aIRR 1.27 (1.07–1.51) [54, 61, 72]. Missed abortion and postpartum depression were not significantly associated with the risk of psoriasis (Fig. 7) [54, 61].
Associations of pregnancy complications with other miscellaneous autoimmune conditions
No significant association was reported with pregnancy complications studied (hyperemesis, ectopic pregnancy, miscarriage, or gestational hypertension) and the development of multiple sclerosis as mentioned in Fig. 7 [64]. The association of postpartum depression and alopecia areata (HR 1.97, 0.72–5.37) [60], and recurrent pregnancy loss and myasthenia gravis (RR 0.85, 0.54–1.31) were not statistically significant [63].
Timing of developing autoimmune diseases following pregnancy/pregnancy complications
Four studies reported the occurrence of autoimmune diseases following pregnancy over different follow-up times [31, 51, 54, 69]. Following a pregnancy complication, a woman’s risk of developing Grave’s disease or SLE was higher in the early years after childbirth in comparison to later in life [35, 39, 54, 63, 69]. For instance, there was a higher risk of SLE in women with pregnancy loss in the first year postpartum IRR 2.64 (1.18–6.29), whereas there was no significant association noted after two or more years postpartum IRR 1.90 (0.87–4.48) [69]. Conversely, the risk of developing rheumatoid arthritis (RR 1.05; 0.98–1.13, 5+ years: RR 2.24; 1.58–3.05, and multiple sclerosis) (RR 1.00; 0.91–1.09, 5+ years: RR 2.20; 1.72–2.77) was greater after 5 or more years postpartum. Also, women with hyperemesis gravidarum were at a greater risk of developing rheumatoid arthritis in the first 4 years post birth; this reduced after 5 years, IRR 1.40 (1.09–1.76) and IRR 1.02 (0.59–1.11), respectively [54, 69]
Discussion
This systematic review provides an overview of the associations of 18 pregnancy complications with the risk of developing 15 autoimmune diseases. This review compiles all the available evidence on pregnancy complications linked to the development of autoimmune diseases in women in later life (Figs. 2, 3, 4, 5, 6 and 7) and generates new evidence by quantitative or qualitative analysis of the studies studying the same exposure and outcomes (Additional file 1 figure 1.10). This also further points out the differences in the results observed in two or more studies analysing the association of the same pregnancy complication and autoimmune disease (Additional file 1 fig. 1.11).
Studies reported associations for gestational hypertension/preeclampsia followed by preterm birth, hyperemesis gravidarum and ectopic pregnancy, and future autoimmunity. However, there was little or no research on some complications such as molar pregnancy or placental disorders. From the perspective of autoimmune disease outcomes, most studies examined the associations with rheumatoid arthritis followed by autoimmune thyroid diseases, Sjögren’s syndrome, and psoriasis. In contrast, there were very few studies that included vitiligo or myasthenia gravis.
Many of the pregnancy complications increased the risk of overall autoimmune diseases almost threefold, particularly hyperemesis gravidarum, miscarriage, gestational hypertension, stillbirth, and antenatal/postpartum depression. Apart from the known association of gestational diabetes and T1DM, results from this review showed that IUGR or stillbirth were associated with almost three- to fourfold increased risk of systemic sclerosis or systemic lupus erythematosus. There was a higher risk of rheumatoid arthritis with preterm birth and low birth weight babies.
There are findings which require further research, for example, the association of miscarriage and development of SLE [58, 65, 69] and the association of gestational hypertension with T1DM [54, 65]. The difference in the findings could possibly be due to the varying study designs or difference in the sample size of the studies. There had been mixed findings amongst the studies included and these may be due to the varying sample size or the study designs.
Earlier studies focused on the association of pregnancy complications with child outcomes such as caesarean birth or pre-eclampsia and the association with long-term health conditions in babies [78,79,80,81,82,83,84]. However, more recently, studies have reported the association of pregnancy complications and the development of long-term conditions in the mother [31, 85]. Reproductive factors and pregnancy complications were found to be associated with later development of metabolic conditions [86,87,88]. An association between pregnancy itself, irrespective of pregnancy complications, and the development of autoimmune diseases has been reported [31]. Some studies identified the association between parity and the development of systemic sclerosis; however, the findings have been conflicting [89,90,91,92].
It is not clear whether the observed pregnancy complications occur in women with preclinical autoimmune disease or whether these events directly pre-dispose to the development of autoimmune disease [93]. In terms of the former, women with undifferentiated connective tissue disease (UCTD), who have features compatible with a CTD but do not have a defined CTD [94], have an increased risk of pregnancy complications including premature delivery, pre-eclampsia, and stillbirth [95]. As approximately 30% of UCTD may progress to CTD, typically SLE, it is possible that some of the pregnancy complications occurred in women who were in the initial stages of UCTD, i.e. the pregnancy complications were due to a subclinical autoimmune disease.
On the other hand, pregnancy/pregnancy complications bring about fluctuations in female sex hormones accompanied by physiological stress [96]. The blood levels of both oestrogen and progesterone increase rapidly from the middle of the second trimester, peaking at term. Oestrogen and progesterone have broad effects on the function of both innate and adaptive immune cells (including monocytes/macrophages, neutrophils, dendritic cells, and T and B lymphocytes) [97]. In pregnancy, placental production of oestriol (E3) increases dramatically. Oestriol has potent anti-inflammatory effects including reducing pro-inflammatory cytokine production, increasing anti-inflammatory cytokines, and reducing CD4+ and CD8+ T cells [98]. Similarly, progesterone increases regulatory T cells and reduces natural killer cell function systemically and within the placenta [98]. It is possible, therefore, that hormonal fluctuations and the loss of this anti-inflammatory state postpartum could accelerate the development of autoimmune disease. Furthermore, oestrogens reduce B cell apoptosis which, whilst contributing to maternal humoral immunity, may promote autoreactive B cell survival and drive the immune system toward autoimmunity [99].
A key driver of future autoimmune disease may be foetal microchimerism [100]. Foetal cells are present at a low frequency in the maternal circulation postpartum and may persist for decades [57, 101,102,103]. Foetal origin microchimerism is observed at increased rates during pregnancy complications such as miscarriage, pre-eclampsia, foetal growth restriction [104], or premature labour [102, 105]. The mechanisms by which foetal microchimeric cells mediate an increased risk of autoimmunity is not understood although an increased number of these cells is observed in the thyroid gland of women with autoimmune thyroid disease [106]. To date, a pathogenic role for foetal microchimeric cells has not been demonstrated, and these cells may induce maternal tolerance to foetal antigens and via a bystander effect reduce the severity of some autoimmune diseases such as RA during pregnancy [106].
Our study has several strengths. The scope of our review was broad and summarises the association of pregnancy complications and the subsequent development of a wide range of autoimmune diseases, and we were able to perform a meta-analysis of studies reporting the same exposure and outcome where possible. We employed rigorous methodology with a pre-specified protocol, and our systematic search was conducted without language restriction and two reviewers screened, extracted data, and appraised the quality of the studies.
There are, however, some limitations. A meta-analysis could not be performed for some of the studies due to missing data such as the sample size or number of exposed/unexposed. Some of the results reported therefore are as reported in one study. Also, nine studies were conducted using the same cohort (Danish birth cohort); this may have a disproportionate effect on our findings. However, efforts were made to avoid duplication in the reporting of results. This study is not able to determine causality and there is a possibility that the women already have undiagnosed preclinical autoimmune diseases, which increased their risk of pregnancy complications in studies, especially those with shorter follow-up time.
Additional research is required that incorporates a comprehensive analysis of pregnancy complications and characterise the phenotype and functionality of persistent foetal origin cells in women with autoimmune diseases compared with healthy women. The exact pathophysiology behind the development of these conditions remains unclear and we do not know why some pregnancy complications have a larger effect than others. To address these questions, prospective longitudinal studies following up on women who experienced pregnancy complications are needed, observing when autoantibodies are first detected [107]. Furthermore, larger epidemiological studies would be required to define whether autoimmune disease is more prevalent in women who have experienced pregnancy complications and if there is a clear underlying association.
Conclusions
This review has reported that there is an association between pregnancy complications and the subsequent development of autoimmune diseases in women. To further address this question, prospective longitudinal studies following up on women who experienced pregnancy complications are needed, observing when autoantibodies are first detected. Meanwhile, clinicians should be vigilant and detect autoimmune conditions early in women with a history of pregnancy complications.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- 95% CI:
-
95% Confidence intervals
- aHR:
-
Adjusted hazard ratio
- aIRR:
-
Adjusted incidence risk ratio
- aOR:
-
Adjusted odds ratio
- aRR:
-
Adjusted risk ratio
- AxSpA:
-
Axial spondyloarthropathy
- CD:
-
Clusters of differentiation
- CS:
-
Caesarean section
- CTD:
-
Connective tissue diseases
- GDM:
-
Gestational diabetes mellitus
- GHT or PE:
-
Gestational hypertension or pre-eclampsia
- HELLP:
-
Haemolysis, elevated liver enzymes, and low platelet syndrome
- IBD:
-
Inflammatory bowel disease
- IUGR:
-
Intrauterine growth retardation
- LBW:
-
Low birth weight
- MESH:
-
Medical subject headings
- MOOSE:
-
Meta-analyses of observational studies
- MS:
-
Multiple sclerosis
- NA:
-
Not applicable
- NOS:
-
Newcastle–Ottawa scale
- PPIE:
-
Patient and public involvement and engagement
- PRISMA:
-
Preferred reporting items for systematic review and meta-analysis
- RA/PSA/ANK S:
-
Rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis
- SGA:
-
Small for gestational age
- SLE:
-
Systemic lupus erythematosus
- T1DM:
-
Type 1 diabetes mellitus
- UCTD:
-
Undifferentiated connective tissue disease
- UK:
-
United Kingdom
- US:
-
United States
References
Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. 2023;80:102266.
Conrad N, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–90.
Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2015;3(4):151–5.
Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–69.
Thomas SL, et al. Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am J Public Health. 2010;100(11):2279–87.
Mallampalli MP, et al. Role of environment and sex differences in the development of autoimmune diseases: a roundtable meeting report. J Womens Health. 2013;22(7):578–86.
Miquel CH, Faz-Lopez B, Guéry JC. Influence of X chromosome in sex-biased autoimmune diseases. J Autoimmun. 2023;137:102992.
Souyris M, et al. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin Immunopathol. 2019;41(2):153–64.
Merrheim J, et al. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun Rev. 2020;19(3):102468.
Nelson JL, et al. Remission of rheumatoid arthritis during pregnancy and maternal-fetal class II alloantigen disparity. Am J Reprod Immunol. 1992;28(3–4):226–7.
Zakarija M, McKenzie JM. Pregnancy-associated changes in the thyroid-stimulating antibody of Graves’ disease and the relationship to neonatal hyperthyroidism*. J Clin Endocrinol Metab. 1983;57(5):1036–40.
Boyd AS, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35(3):169–72.
Khamashta MA, Ruiz-Irastorza G, Hughes GR. Systemic lupus erythematosus flares during pregnancy. Rheum Dis Clin North Am. 1997;23(1):15–30.
Schubert C, et al. Postpartum relapse risk in multiple sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2023;94(9):718–25.
Castles A, et al. Effects of smoking during pregnancy: five meta-analyses. Am J Prev Med. 1999;16(3):208–15.
Cnattingius S, Lambe M. Trends in smoking and overweight during pregnancy: prevalence, risks of pregnancy complications, and adverse pregnancy outcomes. Semin Perinatol. 2002;26(4):286–95. https://doi.org/10.1053/sper.2002.34771.
DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. Eur J Gen Pract. 1995;1(3):117–117.
Meltzer HM, et al. Effect of dietary factors in pregnancy on risk of pregnancy complications: results from the Norwegian Mother and Child Cohort Study. Am J Clin Nutr. 2011;94(suppl_6):1970S-1974S.
Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118(2 Part 1):305–12.
Ramachenderan J, Bradford J, Mclean M. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol. 2008;48(3):228–35.
Jaffar F, Laycock K, Huda MSB. Type 1 diabetes in pregnancy: a review of complications and management. Curr Diabetes Rev. 2022;18(7):e051121197761.
Naik VD, et al. Effects of nutrition and gestational alcohol consumption on fetal growth and development. Nutr Rev. 2022;80(6):1568–79.
Upala S, Yong WC, Sanguankeo A. Association between primary Sjögren’s syndrome and pregnancy complications: a systematic review and meta-analysis. Clin Rheumatol. 2016;35(8):1949–55.
Khizroeva J, et al. Infertility in women with systemic autoimmune diseases. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101369.
Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38(2–3):J266–74.
Bobotsis R, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175(3):464–72.
Arvanitakis K, et al. Adverse pregnancy outcomes in women with celiac disease: a systematic review and meta-analysis. Ann Gastroenterol. 2023;36(1):12–24.
Smyth A, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5(11):2060–8.
Finkelsztejn A, et al. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG. 2011;118(7):790–7.
Wei S, et al. Systemic lupus erythematosus and risk of preterm birth: a systematic review and meta-analysis of observational studies. Lupus. 2017;26(6):563–71.
Khashan AS, et al. Pregnancy and the risk of autoimmune disease. PLoS One. 2011;6(5):e19658.
Jørgensen KT, et al. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. J Autoimmun. 2012;38(2–3):J81–7.
Sobanski V, et al. Special considerations in pregnant systemic sclerosis patients. Expert Rev Clin Immunol. 2016;12(11):1161–73.
Auvinen AM, et al. Type 1 and type 2 diabetes after gestational diabetes: a 23 year cohort study. Diabetologia. 2020;63(10):2123–8.
Hee JY, et al. Pregnancy loss and the risk of rheumatoid arthritis in Chinese women: findings from the China Kadoorie biobank. BMC Public Health. 2022;22(1):1768.
Jorgensen KT, et al. National cohort study of reproductive risk factors for rheumatoid arthritis in Denmark: a role for hyperemesis, gestational hypertension and pre-eclampsia? Ann Rheum Dis. 2010;69(2):358–63.
Gleicher N. Reproductive failure prior to the onset of clinical autoimmune disease. Rheumatology. 1999;38(6):485–7.
Chakravarty E. Pre-disease pregnancy complications and systemic sclerosis: pathogenic or pre-clinical? Arthritis Res Ther. 2012;14(1):102.
Jorgensen KT, et al. Increased risk of rheumatoid arthritis in women with pregnancy complications and poor self-rated health: a study within the Danish National Birth Cohort. Rheumatology. 2014;53(8):1513–9.
Ma KK, et al. Adverse pregnancy outcomes and risk of subsequent rheumatoid arthritis. Arthritis Rheumatol. 2014;66(3):508–12.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
Team TE. EndNote, Philadelphia,PA. Clarivate. EndNote 20. 64 bit. 2013.
Covidence Systematic Review Software. Veritas Health Innovation, Melbourne Australia. www.covidence.org. Accessed July 2024.
Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. Available from https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-01.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta analysis. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Assessed on Nov 2023.
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100.
StataCorp. Stata statistical software: release 17. College Station: StataCorp LLC; 2021.
RStudio Team. RStudio: integrated development for R. Boston: RStudio, Inc.; 2019. http://www.rstudio.com/.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020. https://www.R-project.org/.
Bergink V, et al. Prevalence of autoimmune thyroid dysfunction in postpartum psychosis. Br J Psychiatry. 2011;198(4):264–8.
Bergink V, et al. Comorbidity of autoimmune thyroid disorders and psychiatric disorders during the postpartum period: a Danish nationwide register-based cohort study. Psychol Med. 2018;48(8):1291–8.
Hardy C, et al. Pregnancy outcome and family size in systemic lupus erythematosus: a case-control study. Rheumatology (Oxford). 1999;38(6):559–63.
Harpsoe MC, et al. Risk of inflammatory bowel disease according to self-rated health, pregnancy course, and pregnancy complications: a study within the Danish National Birth Cohort. PLoS One. 2013;8(3):e59698.
Jørgensen KT, et al. Hyperemesis, gestational hypertensive disorders, pregnancy losses and risk of autoimmune diseases in a Danish population-based cohort. J Autoimmun. 2012;38(2):J120–8.
Julkunen H, et al. Fetal outcome in lupus pregnancy: a retrospective case-control study of 242 pregnancies in 112 patients. Lupus. 1993;2(2):125–31.
Kamper-Jorgensen M, Gammill HS, Nelson JL. Preeclampsia and scleroderma: a prospective nationwide analysis. Acta Obstet Gynecol Scand. 2018;97(5):587–90.
Kay A, Bach F. Subfertility before and after the development of rheumatoid arthritis in women. Ann Rheum Dis. 1965;24(2):169–73.
Kither H, et al. Adverse pregnancy outcomes and subsequent development of connective tissue disease in the UK: an epidemiological study. BJOG. 2020;127(8):941–9.
Lee KA, et al. Pregnancy-associated risk factors and incidence of systemic sclerosis in primiparous women: a nationwide population-based cohort study. Mod Rheumatol. 2022;32(1):149–54.
Lin CY, et al. Postpartum depression and subsequent autoimmune diseases in Taiwan. Int J Environ Res Public Health. 2018;15(8):20.
Lin LT, et al. Increased risk of systemic lupus erythematosus in pregnancy-induced hypertension: a nationwide population-based retrospective cohort study. Medicine. 2016;95(30):e4407.
Mao Y, et al. Association between history of gestational diabetes mellitus and the risk of arthritis in women. Front Public Health. 2022;10:878845.
Mikkelsen AP, et al. Pregnancy loss and risk of multiple sclerosis and autoimmune neurological disorder: a nationwide cohort study. PLoS One. 2022;17(3):e0266203.
Nielsen NM, et al. Reproductive history and risk of multiple sclerosis. Epidemiology. 2011;22(4):546–52.
Savitz DA, et al. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180(1):41–4.
Siamopoulou-Mavridou A, et al. Outcome of pregnancy in patients with autoimmune rheumatic disease before the disease onset. Ann Rheum Dis. 1988;47(12):982–7.
Spector TD, Silman AJ. Is poor pregnancy outcome a risk factor in rheumatoid arthritis? Ann Rheum Dis. 1990;49(1):12.
Stuart AE, Amer-Wahlin I, Kallen KBM. Neonatal delivery weight and risk of future maternal diabetes. Int J Gynaecol Obstet. 2018;140(1):111–7.
Ulff-Møller CJ, et al. Reproductive factors and risk of systemic lupus erythematosus: nationwide cohort study in Denmark. J Rheumatol. 2009;36(9):1903–9.
van Wyk L, et al. Increased incidence of pregnancy complications in women who later develop scleroderma: a case control study. Arthritis Res Ther. 2011;13(6):R183.
Wallenius M, et al. Pregnancy and delivery in women with chronic inflammatory arthritides with a specific focus on first birth. Arthritis Rheum. 2011;63(6):1534–42.
WikstromShemer EA, et al. Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: a population-based cohort study. J Hepatol. 2015;63(2):456–61.
Brann E, et al. Bidirectional association between autoimmune disease and perinatal depression: a nationwide study with sibling comparison. Mol Psychiatry. 2024;09:09.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane; 2023. Available from www.training.cochrane.org/handbook.
Tramèr MR, et al. Impact of covert duplicate publication on meta-analysis: a case study. BMJ. 1997;315(7109):635–40.
von Elm E, et al. Different patterns of duplicate publication: an analysis of articles used in systematic reviews. JAMA. 2004;291(8):974–80.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Bi S, et al. Long-term effects of preeclampsia on metabolic and biochemical outcomes in offspring: what can be expected from a meta-analysis? Obes Rev. 2022;23(5):e13411.
Black M, et al. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314(21):2271–9.
Bruce A, Black M, Bhattacharya S. Mode of delivery and risk of inflammatory bowel disease in the offspring: systematic review and meta-analysis of observational studies. Inflamm Bowel Dis. 2014;20(7):1217–26.
Cardwell CR, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53(4):641–51.
Dalla Costa G, et al. Caesarean section and infant formula feeding are associated with an earlier age of onset of multiple sclerosis. Mult Scler Relat Disord. 2019;33:75–7.
Nielsen NM, et al. Maternal diabetes and risk of multiple sclerosis in the offspring: a Danish nationwide register-based cohort study. Mult Scler. 2021;27(11):1686–94.
Nakamura N, et al. Mortality and neurological outcomes in extremely and very preterm infants born to mothers with hypertensive disorders of pregnancy. Sci Rep. 2021;11(1):1729.
Jones WR. Autoimmune disease and pregnancy. Aust N Z J Obstet Gynaecol. 1994;34(3):251–8.
Neiger R. Long-term effects of pregnancy complications on maternal health: a review. J Clin Med. 2017;6(8):76.
Panaitescu AM, et al. Pregnancy complications can foreshadow future disease-long-term outcomes of a complicated pregnancy. Medicina (Kaunas). 2021;57(12):1320.
Okoth K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502.
Cockrill T, Del Junco DJ, Arnett FC, Assassi S, Tan FK, McNearney T, et al. Separate influences of birth order and gravidity/parity on the development of systemic sclerosis. Arthritis Care Res. 2010;62(3):418–24. https://doi.org/10.1002/acr.20096.
Pisa FE, et al. Reproductive factors and the risk of scleroderma: an Italian case-control study. Arthritis Rheum. 2002;46(2):451–6.
Russo PA, Lester S, Roberts-Thomson PJ. Systemic sclerosis, birth order and parity. Int J Rheum Dis. 2014;17(5):557–61.
Lambe M, et al. Childbearing and the risk of scleroderma: a population-based study in Sweden. Am J Epidemiol. 2004;159(2):162–6.
Beneventi F, et al. Impact of pregnancy on progression of preclinical autoimmune disorders: a prospective cohort study. Rheumatology. 2022;62(9):2971–8.
Antunes M, et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open. 2018;4(Suppl 1):e000786.
Spinillo A, et al. The effect of newly diagnosed undifferentiated connective tissue disease on pregnancy outcome. Am J Obstet Gynecol. 2008;199(6):632.e1-6.
Assad S, et al. Role of sex hormone levels and psychological stress in the pathogenesis of autoimmune diseases. Cureus. 2017;9:e1315. https://doi.org/10.7759/cureus.1315.
Serena C, et al. Undifferentiated connective tissue disease in pregnancy: a topic yet to be explored. Front Pharmacol. 2022;13:820760.
Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–71.
Ding J, Zhu BT. Unique effect of the pregnancy hormone estriol on antigen-induced production of specific antibodies in female BALB/c mice. Steroids. 2008;73(3):289–98.
Ubeda F, Wild G. Microchimerism as a source of information on future pregnancies. Proc R Soc Lond B Biol Sci. 2023;290(2005):20231142.
Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol. 2012;33(8):421–7.
Bianchi DW, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705–8.
Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest. 2008;37(5–6):631–44.
Al-Mufti R, et al. Fetal cells in maternal blood of pregnancies with severe fetal growth restriction. Hum Reprod. 2000;15(1):218–21.
Leung TN, et al. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352(9144):1904–5.
Fugazzola L, Cirello V, Beck-Peccoz P. Microchimerism and endocrine disorders. J Clin Endocrinol Metab. 2012;97(5):1452–61.
Luiro K, et al. Autoantibodies predict type 1 diabetes after gestational diabetes - a 23-year cohort study. Front Endocrinol. 2023;14:1286375.
Acknowledgements
MuM-PreDiCT consortium
Funding
This work was funded by the Strategic Priority Fund ‘Tackling multimorbidity at scale’ programme (grant number-MR/W014432/1) delivered by the Medical Research Council and the National Institute for Health and Care Research in partnership with the Economic and Social Research Council and in collaboration with the Engineering and Physical Sciences Research Council.
Author information
Authors and Affiliations
Consortia
Contributions
MS was responsible for the analysis and drafting of the manuscript. FF/JW were the second reviewers for the study selection, data extraction check, and quality appraisal. FC and KN were the third reviewers and provided their inputs and guidance at each step of the review. KN, JR, AS, FC, and SW were responsible for revising the manuscript critically and for important intellectual content. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @Nirantharakumar (Krishnarajah Nirantharakumar), @StevenWambua (Steven Wambua), @meghasingh_16 (Megha Singh)
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since this review analyses the data from the prior systematic reviews, no ethical approval is required. Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
Co-author AS was part of university of Birmingham when this review was initiated but moved to work with Astrazeneca. She currently holds an honorary contract with the University of Birmingham. All the other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3550_MOESM1_ESM.docx
Additional file 1: Table 1. Search Strategy MEDLINE. Table 2. The Preferred Reporting Items for Systematic reviews and Meta-Analyseschecklist. Table 3. List of excluded studies. Table 4.1. Quality assessment of the Cohort studies using NOS. Table 4.2. Quality assessment of the case control studies using NOS. Table 4.3. Quality assessment of the cross-sectional studies using NOS. Figure 1.1. Meta-analysis of two studies reporting association of miscarriage and future development of SLE. Figure 1.2. Meta-analysis of two studies reporting association of miscarriage and future development of rheumatoid arthritis. Figure 1.5. Meta-analysis of two studies reporting association of stillbirth and future development of SLE. Figure 1.6. Meta-analysis of two studies reporting association of gestational hypertension or pre-eclampsia and future development of SLE. Figure 1.7. Meta-analysis of two studies reporting association of preterm birth and future development of SLE. Figure 1.8. Meta-analysis of two studies reporting association of preterm birth and future development of rheumatoid arthritis. Figure 1.9. Meta-analysis of two studies reporting association of gestational diabetes and future development of T1DM. Figure 1.10. The new findings from this review. Figure 1.11. The mixed findings of this review. Table 5. Cohort studies with same or overlapping cohorts. Table 6. Data Extraction form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Singh, M., Fayaz, F.F.A., Wang, J. et al. Pregnancy complications and autoimmune diseases in women: systematic review and meta-analysis. BMC Med 22, 339 (2024). https://doi.org/10.1186/s12916-024-03550-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03550-5